Figure 6. Gap junctions are essential to amplify inflammation and bystander apoptosis induced by the few HIV-infected astrocytes.
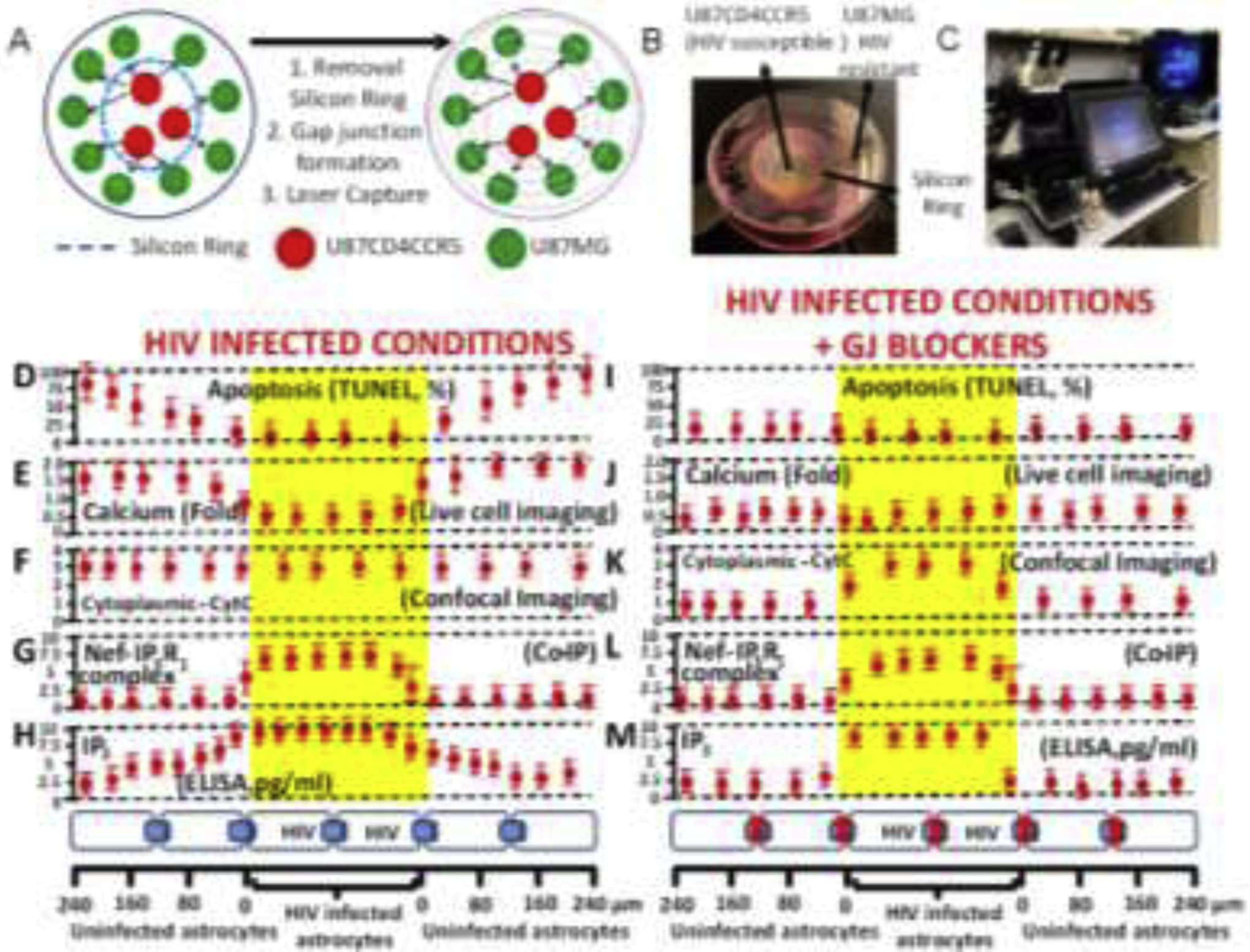
(A) A co-culture model between U87MG (resistant to HIV infection) and U87CD4CCR5 (sensitive to HIV infection) was used in the following experiments. The cell types are divided by a silicon ring that, upon removal, enables both cell types to establish gap junctional communication. This model provided the necessary spatial resolution to examine apoptosis and distance from HIV-infected into uninfected cells. (B) Representative picture of the model used. (C) Equipment used for the concentric laser capture is described below. (D to H) represent cocultures of both cell types in HIV-infected conditions with functional GJ communication (see bottom of the figure to see cell distribution and distances examined). (D) Quantification of apoptosis versus distance from HIV-infected cells. Apoptosis was only detected in uninfected cells (primary or U87MG cells). (E) Levels of intracellular calcium as determined by calcium imaging using fura-2. Data are expressed in folds as compared to control conditions. Calcium increased mostly in uninfected cells. (F) Quantification of CytC in the cytoplasm or “leak” of CytC from the mitochondria into the cytoplasm using confocal microscopy for mitotracker, DAPI, and CytC. CytC was present in the cytoplasm of HIV-infected and uninfected cells. (G) Co-immunoprecipitation of the laser captured material to examine the interaction between Nef and IP3 receptors 1, 2, or 3. Only IP3R1/3 interacted with Nef, but only in the HIV-infected astrocytes. (H) ELISA of unfixed laser captured material to quantify the amount of IP3. IP3 was high in HIV-infected astrocytes, and IP3 concentration was reduced in a distance-dependent manner in uninfected astrocytes. (I–M) correspond to the same set of experiments shown in D to H in the presence of the gap junction blocker, AGA. Functional GJ communication is required for bystander apoptosis of uninfected cells, calcium increase in uninfected cells (compare E versus J), the release of CytC into the cytoplasm in uninfected cells (compare F versus K), the formation of the complex between Nef and IP3R1 was not affected in HIV-infected astrocytes (compare G versus L), and IP3 did not diffuse into uninfected astrocytes (compare H versus M). Overall, these data indicate that HIV-infected astrocytes are protected from apoptosis by altering the calcium/IP3 axis and probably by generating specific interactions between viral proteins and the ER receptors. In future work, we will expand our study of this critical point in the survival of HIV-infected astrocytes. All data points are represented as mean ± SD. Each point corresponds to the data integration of an area of 25±8.98 μm. n=4 independent experiments.
