Fig. 1.
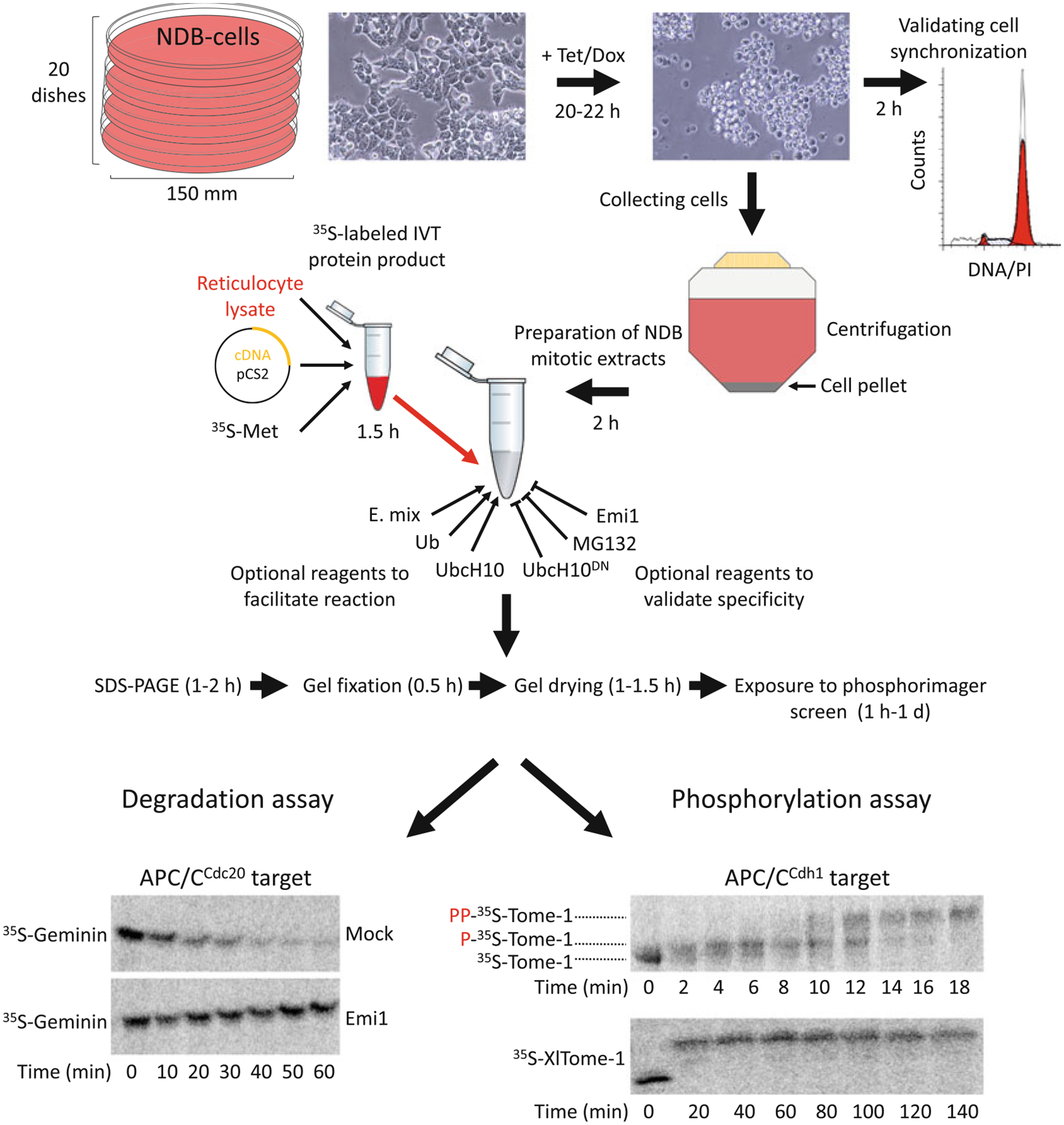
NDB cell- and cell-free systems for elucidating human mitosis. (a) NDB cell system is based on human 293-T-REx™ cells stably expressing nondegradable (ND) mutant of Cyclin B1 under a tetracycline (Tet)/doxycycline (Dox)-regulated CMV promoter. Expression of ND-Cyclin B1 induces mitotic arrest in an anaphase-like state. The constant activity of the Cdk1–Cyclin B1 complex prevents mitotic exit and maintains a peak level of APC/CCdc20 activity for hours. NDB extracts are typically prepared from 20 plates of 150 mm/diameter. Recommended cell confluency for Tet-induced expression is 75–80%. Following 20–22 h treatment with Tet, cells acquire a spherical shape. This archetypal morphology indicates mitotic arrest. Cell synchronization should be further validated by standard DNA quantification. The arrested cells will be loosely attached to the surface, if at all. Thus, cells can be collected directly by gentle pipetting (without trypsinization) into a single 500 mL conical tube. Once cells are pelleted and washed, they are ready for extract preparation. In vitro translated (IVT) protein products are generated in reticulocyte lysate supplemented with radiolabeled methio-nine (35S-Met). pCS2-based expression vectors and reticulocyte lysates supporting transcription from SP6 promoter are recommended. A typical reaction mix contain 10–20 μL NDB mitotic extracts and 0.5–1.5 μL IVT product. Extracts already contain energy-regeneration mix (E-mix), as well as endogenous Ubiquitin (Ub) and the E2 enzyme UbcH10. As such, extracts are highly active. Yet adding 0.5–1 μL of ×20 E. mix, recombinant Ub, and UncH10 into the reaction mix can facilitate proteolysis mediated by APC/CCdc20. Conversely, proteasome inhibitors (e.g., MG132), dominant negative UbcH10 (UbcH10DN), or APC/C specific inhibitors (e.g., Emi1 or TAME) can be added for validating the potency and specificity of the assay. Assays are typically performed in a temperature range of 23–30 °C. During degradation/mobility-shift assays, 3–5 μL of the reaction mix is sampled each time point, mixed with Laemmli buffer, boiled and frozen at 80 °C. Protein sampled are resolved by SDS-PAGE. Gels are then soaked in a distain solution, heat/vacuum dried, and exposed to a Phosphorimager screen for 1 h to 1 day. Longer exposure might be helpful in case of weak signals. The potency and specificity of NDB mitotic extracts is demonstrated; Geminin, an APC/CCdc20 substrate, but not Tome-1, an APC/CCdh1 substrate, is degraded. This degradation is blocked by Emi1. Orderly phosphorylation of Tome-1 is evident in minute-scale resolution by a gradual mobility shift. The high mobility shift of Tome-1 observed after 140 min indicates for the stability and potency of this cell-free system
