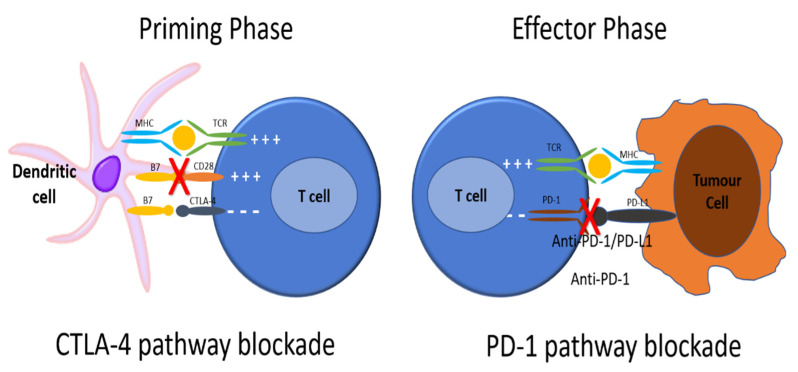
Figure 1.
To be activated, T cells require major histocompatibility complex (MHC) class II molecules on an antigen presenting cell (APC) to present an antigen (Ag) that is recognized by the T cell receptor. Next, the CD28 receptor on the T cell is bound by CD80/86 on the APC, signaling the T cell to be activated. CTLA4 is found on T cell surfaces and competes with CD28 for binding to CD80/86 on the APC. When this interaction predominates, T cell activation signaling is attenuated. As well, PD-1 receptors are expressed on the surface of T cells. When PD-1 receptors are engaged by programmed death ligand 1 (PD-L1) on an APC, the T cell that recognizes the Ag being presented by the APC activates signaling pathways that downregulate activation and promote apoptosis. There is also reduced apoptosis of T-regulatory cells (Treg), facilitating downregulation of the immune response to that antigen. Cancer cells exploit these important physiologic mechanisms by upregulating PD-L1 expression on their cell surface, thereby attenuating any anti-tumor immune response through inducing the quiescence of tumor-reactive T cells. ICIs such as anti-CTLA-4, anti-PD-1, and anti-PD-L1 antibodies have been developed to restore the immune system’s ability to mount an anti-tumor immune response. Anti-CTLA-4 antibodies block the interaction between CTLA-4 and CD80/86 on the APCs, allowing for increased T cell activation. Anti-PD-1 and anti-PD-L1 antibodies block the interaction between PD-1 and PD-L1, allowing for increased T cell activation to Ag being presented by the tumor cell. In this way, the tumor cell is no longer recognized as “self”, tumor-reactive T cells are no longer shunted towards quiescence, and an anti-tumor immune response can be mounted.