Cytokines have far-reaching effects on the behavior of immune cells. Given their powerful roles, there has been a long history of trying to harness cytokines as therapeutic drugs for cancer and other diseases. However, there are several problems that severely limit the therapeutic use of cytokines, including their pleiotropic actions and systemic toxicity. Overcoming these issues to create the next generation of cytokine-based therapies will require sophisticated control over their spatial-temporal function. New approaches in protein and cell engineering are emerging that allow distinct and multiple levels at which to program cytokine regulation—from engineering individual cytokines, to cytokine-receptor pairs, and ultimately, more complex cytokine-sensing, -secreting, and -consuming cell circuits. These technologies may confer the ability to precisely sculpt the local cytokine environment, and by doing so, improve the potency of cytokine drugs and deepen our understanding of the language of cytokine communication.
The biological function of cytokines is broad, encompassing immune cell proliferation, death, activation, and inhibition. The effects of these secreted signaling molecules depends on their local concentration, which is driven by the rates of cytokine production, diffusion, and consumption. Cytokine-mediated cell-cell communication can be autocrine, paracrine, or endocrine. Together, these core features of cytokine communication are thought to shape the ecosystem of specific tissues or tumors. Perhaps most notable is how this set of secreted factors can achieve such diverse yet highly spatially coordinated physiological outcomes within the complex environment of the body.
Interleukins and interferons are cytokines that have clinical relevance in cancer. Direct infusion of cytokines into a tissue can have potent therapeutic effects—killing transformed cells in a tumor or stimulating the expansion and cytotoxic activities of host or adoptively transferred immune cells. So far, two cytokine drugs [interferon-α (IFN-α) and interleukin-2 (IL-2)] have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of hairy cell leukemia, melanoma, and other cancers.
Nonetheless, there are fundamental problems that severely limit the therapeutic use of natural cytokines: short circulation half-life, off-target effects, and inherent pleiotropic functions. Clinically, repeated systemic administration of IL-2 at high doses is typically needed to achieve therapeutic response as a result of its short circulation half-life (the serum half-life of IL-2 is ~90 min). Most seriously, cytokines act as a double-edged sword—they target many cell types. Thus, for example, high dosing regimens of IL-2 elicit severe systemic toxicity because the cytokine accumulates not only in the disease tissue, but also in healthy bystander organs, where IL-2 induces severe adverse effects including vascular leak syndrome and pulmonary edema (1). IL-2 causes many changes in immune cells, some that may be desired and some that are therapeutically detrimental. IL-2 acts on multiple immune cells—it drives proliferation of effector T cells, but also stimulates T regulatory cells (Treg) that cause suppressive outcomes. Treg stimulation can promote tumor growth by serving as an IL-2 cytokine sink to deplete the growth factor necessary for effector T cell–mediated antitumor activity, and by directly disarming effector T cells.
Much of the existing efforts to engineer improved cytokines have focused on IL-2 because of its long history as a cancer therapeutic target. A more-conventional chemical strategy is to attach IL-2 to moieties such as polyethylene glycol (PEG) to extend its serum half-life. PEGylating IL-2 creates an IL-2 pro-drug that mitigates rapid systemic activation upon administration by hindering receptor binding. Once the PEG is slowly released from the prodrug, the active free IL-2 becomes bio-available over time (2). This modified IL-2 showed significantly longer serum half-life and was well tolerated in recent phase 1 trials in patients with advanced solid tumors (NCT02983045). Similarly, a PEGylated form of IFN-α showed longer half-life, and was approved by the FDA for the treatment of melanoma. Nonetheless, current evidence suggests that these approaches do not sufficiently address the major challenges of systemic toxicity and pleiotropic action.
Creating the next generation of cytokine-based therapies that address pleiotropic toxicity will require far greater control over cytokine function. Advances in protein and cell engineering are emerging that provide multiple new levels at which to program the time and space of cytokine-driven immune responses (see the figure). Protein engineering and screening have allowed investigators to more rationally engineer synthetic cytokines with selective bias toward a desired function. Pioneering studies using phage display screens created a human growth hormone (hGH) mutant that bound ~400 fold more tightly to its receptor than the wild-type form (3). Following this example, most cytokine engineering strategies use a combination of directed mutagenesis and library-based screens. For instance, an IL-2 mutant (BAY 50–4798) with reduced affinity for IL-2 receptor-β (IL-2Rβ) showed preferential activation for T cells over natural killer (NK) cells (which can cause toxicity) 3000-fold higher than the wild-type IL-2 (4). Even though this mutant was shown to be less toxic when tested in preclinical models, phase 1 trials in patients with metastatic melanoma or renal cancer failed to show significant benefit or reduction in side effects over IL-2 (5), likely because multiple IL-2–responsive populations can contribute to toxicity.
Figure. Engineering cytokine communication.
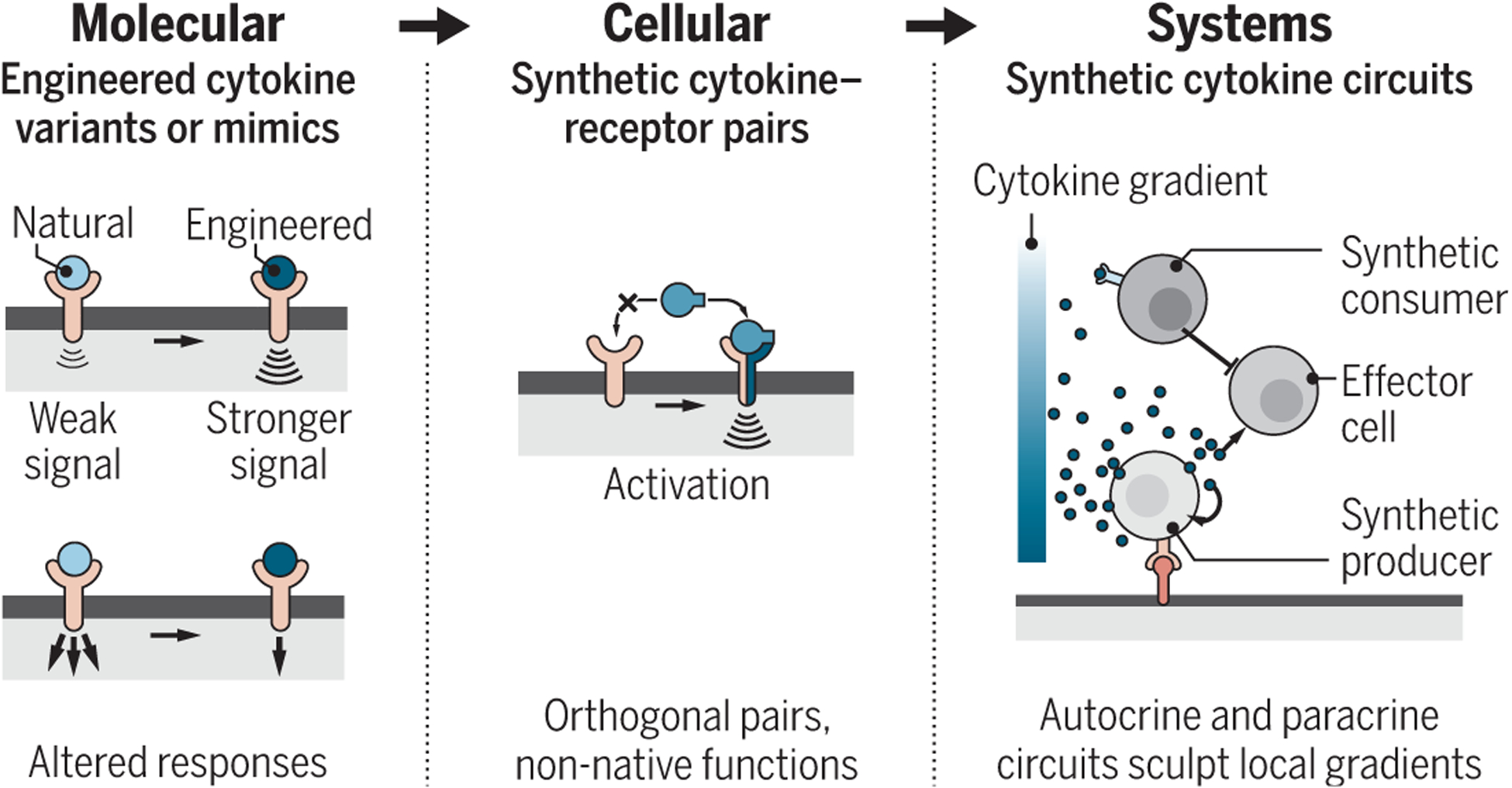
Emerging protein- and cell-engineering technologies may provide multiple levels at which to program cytokine-driven immune responses. These tools may lead to powerful therapeutics and improve understanding of cytokine-based communication.
In a different approach, a superagonist form of IL-2, called “Super2,” was engineered to have increased binding affinity for IL-2Rβ, rationalizing that it would preferentially trigger naïve T cells that are otherwise insensitive to IL-2 owing to their low expression of IL-2Rα (which stabilizes IL-2 interaction with IL-2Rβ). Indeed, Super2 showed superior expansion of cytotoxic T cells relative to regulatory T cells than did IL-2 and also reduced pulmonary toxicity in preclinical tumor models (6). Building on this work, an entirely new cytokine termed “neo-2/15” was designed in silico that signals through the shared chains of IL-2 and IL-15 receptors (the heterodimer of IL-2Rβ and IL-2Rγc) but has no binding sites for their respective private chains (IL-2Rα and IL-15Rα). Bypassing the private receptors allows neo-2/15 to preferentially signal to antitumor lymphocytes. In preclinical tumor models, neo-2/15 shows superior therapeutic activity to IL-2 and reduced toxicity (7). Recent efforts in cytokine engineering have also resulted in a “decoy-resistant” IL-18 (DR-18), which maintains native IL-18 signaling but is impervious to inhibition by IL-18 binding peptide (IL-18BP), an endogenous secreted antagonist for wild-type IL-18 (8). Unlike IL-18, DR-18 showed effective antitumor effects in mice resistant to immune-checkpoint therapies. Clinical examples of designer cytokines include Pitrakinra, an engineered IL-4 variant that acts as an antagonist. In completed phase 2 trials, Pitrakinra showed some benefits for treating IL–4-associated asthma, with fewer adverse events (9).
A more radical emerging approach to limiting detrimental cytokine action is to engineer orthogonal cytokine-receptor pairs. This approach entails changing both the cytokine molecule and the way a target cell recognizes the engineered cytokine—an approach that fits well with engineered immune cell therapies [such as adoptive transfer of chimeric antigen receptor (CAR) T cells], which already involves a commitment to engineering a target effector immune cell. For example, to precisely target IL-2 functions to specific target T cells, an orthogonal IL-2/IL-2R pair (ortho2 and ortho2R, respectively) was developed (10). Ortho2 is a mutant IL-2 that can no longer bind to the native IL-2R; similarly, ortho2R is a mutant IL-2R that does not recognize the native IL-2. The ortho2/2R pair are engineered to only interact with each other. Thus, ortho2 stimulates only the complementary T cells that have been engineered to express ortho2R. Although engineering perfect orthogonal pairs with wild-type like potency remains a challenge, this pioneering work shows the power of the approach. In mouse models, ortho2 cytokine-receptor pairs show a high degree of specificity and orthogonality in vivo, suggesting that ortho2 may be a powerful tool to precisely control the proliferation of engineered cells while remaining inert to the endogenous immune system. This concept can be broadly applied to other cytokines and could be used to control CAR T cells or any other engineered therapeutic cell.
Moving beyond cytokines that already exist in nature, non-natural cytokines, or “synthekines,” have also been described (11). These synthekines do not bind to natural cytokine receptor pairings, but instead assemble non-natural receptor heterodimers that lead to previously undescribed responses. Together, these important advances demonstrate the possibility of going beyond the proteins that our genomes naturally encode and open exciting therapeutic opportunities.
An even higher level of emerging engineering involves the creation of new multicellular cytokine systems and circuits. The highly localized action of cytokines originates from the ability of specific cells to read local signals that control both the production and consumption of cytokines—in essence, the immune system sculpts spatial gradients and niches using source and sink cells (in addition to effector cells that read the gradients) (12). With our mechanistic understanding of cellular biology and cell-cell communication, it may now be possible to rationally sculpt cytokine gradients, using cells that are synthetically engineered to act as sources and sinks. Engineering such gradients will likely require dynamic and discrete combinations of agonists and antagonists in the forms of cytokines, inhibitors, and cytokine receptors.
An early approach to engineering “source cells” has been to design CAR T cells to express proinflammatory cytokines (e.g., IL-12), either constitutively or under a CAR-controlled promoter (13). Engineering of cytokine consuming “sink” cells can also be a complementary powerful tool for sculpting cytokine milieus. A recent example of this nascent concept is engineered T cells constitutively expressing a nonsignaling membrane-bound IL-6R to effectively deplete IL-6 and thus reduce IL-6–mediated toxicity in mice (14). More controlled approaches are emerging in which modular sensing receptors, such as synNotch receptors (15), can be used to induce cytokine secretion or consumption in response to local disease or tissue antigen signals, yielding the potential of highly localized and programmable sink or source cells. Such engineered cellular delivery systems may offer one of the best ways to autonomously target and modulate local disease environments (including metastases) to drive antitumor responses and to remodel immunosuppressive responses, especially when combined with engineered autocrine or paracrine signaling that can locally amplify activity through positive feedback. Conversely, similar approaches could be used to create locally suppressed micro-environments in the case of autoimmunity.
These concepts are still at an early stage, and much experimental and theoretical validation are needed before they can reach the clinic. As a therapy, it is also important to critically evaluate the timing of intervention during disease progression. Ultimately, these multicellular cytokine control circuits may allow modulation of the expansion and death of engineered and host cells, and tuning the amplitude and duration of cytokines in a precisely targeted local environment. The future for engineered cytokines and cellular circuits is promising given that they could have many advantages compared to current cytokine therapies, including higher specificity, local and tissue-specific actions, and reduced off-target effects. It is expected that these strategies will be broadly impactful in treating other diseases involving inflammatory imbalances, such as autoimmunity, fibrosis, and tissue or wound regeneration. As more attempts are made to sculpt local cytokine microenvironments, deeper understanding of the language and grammar of cytokine-based communication will be gained.
ACKNOWLEDGMENTS
The authors are supported by the Howard Hughes Medical Institute (W.A.L.), the NIH (R01CA196277, P50GM081879, UC4DK116264, U54CA244438), and the Cancer Research Institute (A.W.L.). Thanks to members of the Lim lab and H. El-Samad. W.A.L is adviser to Allogene, a shareholder of Gilead, and has applied for patents on cytokine delivery circuits. A.W.L. is an employee of Lyell.
REFERENCES AND NOTES
- 1.Klapper JA et al. , Cancer 113, 293 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charych DH et al. , Clin. Cancer Res 22, 680 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Lowman HB, Wells JA, J. Mol. Biol 234, 564 (1993). [DOI] [PubMed] [Google Scholar]
- 4.Shanafelt AB et al. , Nat. Biotechnol 18, 1197 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Margolin K et al. , Clin. Cancer Res 13, 3312 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Levin AM et al. , Nature 484, 529 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DSilva D-A et al. , Nature 565, 186 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou T et al. , Nature 583, 609 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M, Lancet 370, 1422 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Sockolosky JT et al. , Science 359, 1037 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moraga I et al. , eLife 6, e22882 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oyler-Yaniv A et al. , Immunity 46, 609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeku OO, Purdon TJ, Koneru M, Spriggs D, Brentjens RJ, Sci. Rep 7, 10541 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan AHJ, Vinanica N, Campana D, Blood Adv. 4, 1419 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roybal KT et al. , Cell 167, 419 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


