Abstract
INTRODUCTION:
Biologics, such as tumor necrosis factor inhibitors, anti-integrins and anticytokines, are therapies for inflammatory bowel disease (IBD) that may increase the risk of infection. Most biologics undergo placental transfer during pregnancy and persist at detectable concentrations in exposed infants. Whether this is associated with an increased risk of infantile infections is controversial. We performed a systematic review and meta-analysis evaluating the risk of infantile infections after in utero exposure to biologics used to treat IBD.
METHODS:
We searched PubMed, Embase, Scopus, Web of Science, and CENTRAL from inception to June 2020 to evaluate the association of biologic therapy during pregnancy in women with IBD and risk of infantile infections. Odds ratios of outcomes were pooled and analyzed using a random effects model.
RESULTS:
Nine studies met the inclusion criteria comprising 8,013 women with IBD (5,212 Crohn’s disease, 2,801 ulcerative colitis) who gave birth to 8,490 infants. Biologic use during pregnancy was not associated with an increased risk of all infantile infections (odds ratio [OR] 0.91, 95% confidence interval [CI] 0.73–1.14, I2 = 30%). In a subgroup analysis for the type of infection, biologic use was associated with increased infantile upper respiratory infections (OR 1.57, 95% CI 1.02–2.40, I2 = 4%). Biologic use during pregnancy was not associated with infantile antibiotic use (OR 0.91, 95% CI 0.73–1.14, I2 = 30%) or infection-related hospitalizations (OR 1.33, 95% CI 0.95–1.86, I2 = 26%).
DISCUSSION:
Biologics use during pregnancy in women with IBD is not associated with the overall risk of infantile infections or serious infections requiring antibiotics or hospitalizations but is associated with an increased risk of upper respiratory infections.
INTRODUCTION
Inflammatory bowel disease (IBD) is increasing worldwide and is associated with significant healthcare utilization and suboptimal quality of life (1). The incidence of IBD is highest among women of reproductive age, with 25% of women becoming pregnant after diagnosis (2). Among women with IBD, active disease is associated with an increased risk of pregnancy complications and adverse outcomes (3). Although many studies have investigated the frequency of adverse pregnancy outcomes attributable to a variety of IBD therapies, the magnitude of these effects as they relate to infantile infections subsequent to exposed pregnancies remains a topic of debate (4).
Immunosuppressive medications are a mainstay of treatment for IBD, and biologic therapies such as monoclonal antibodies that abrogate tumor necrosis factor (TNF) activity increasingly form the backbone of management (5). The introduction of a variety of novel biologic therapies such as those targeting the integrin α4β7 (vedolizumab) (6) and p40 subunit of IL-12/IL-23 (ustekinumab) (7) have expanded the armamentarium of IBD therapies and led to a dramatic increase in the proportion of patients with controlled disease (8). Although biologics are effective treatments for IBD, their immunosuppressive effects increase the risk of infection (9–11).The risk of infection in infants exposed to biologics during pregnancy is of particular concern for patients and clinicians.
A broad array of biologics used to treat IBD have been detected in infants, with some persisting for up to 1 year through transplacental transfer in utero (12). Data regarding the risk of infantile infections after in utero exposure to biologic therapy are conflicting. A widely cited case report (13) demonstrated a fatal case of disseminated mycobacterial infection after BCG vaccination in an infant born to a mother with Crohn’s disease treated with infliximab. Another study (12) showed that infants born to mothers treated with concomitant TNF inhibitor and thiopurine therapy during pregnancy had a 3-fold increased risk of infantile infection compared with anti-TNF monotherapy. By contrast, a large cohort study of patients with IBD (14) found that biologics during pregnancy were associated with an increased risk of maternal, but not infantile, infections. In light of these conflicting data, some clinicians turned to certolizumab, a monovalent Fab’ fragment incapable of crossing the placental barrier (15). Although certolizumab may be a more appealing therapy during pregnancy in IBD, a previous network meta-analysis showed that infliximab and adalimumab are more effective than certolizumab in induction and maintenance therapy in IBD (16). To address these conflicts and to better guide clinicians and patients, we performed a systematic review and meta-analysis to quantify the subsequent risk of infantile infections after fetal exposure to biologics.
METHODS
Study protocol
Our systematic review and meta‐analysis was conducted according to the MOOSE (17) guidelines (see Supplementary Table 2, Supplementary Digital Content 2, http://links.lww.com/AJG/B753, MOOSE checklist), reported according to the PRISMA guideline (18), and was preregistered at the PROS-PERO Database (http://www.crd.york.ac.uk/PROSPERO) Reg. No. CRD42019135721. We performed a search of major electronic databases from inception to June 2020 including (i) MEDLINE (PubMed), (ii) EMBASE, (iii) Scopus, (iv) Web of Science, and (v) CENTRAL (Cochrane Central Register of Controlled Trials). The following research strategy was performed in MEDLINE and adapted to the other databases: (“Inflammatory Bowel Diseases” [MeSH] OR Inflammatory Bowel Disease*[TIAB] OR Crohn*[TIAB] OR Ulcerative Colitis*[TIAB] OR IBD[TIAB] OR Proctocolitis*[TIAB] OR Proctosigmoiditis*[TIAB] OR Rectocolitis*[TIAB] OR Rectosigmoiditis*[TIAB] OR Proctitis*[TIAB]) OR “Pregnancy”[-MeSH] OR Pregnanc*[TIAB] OR new-born*[TIAB] OR Lactation*[TIAB] OR “Infant”[MeSH] OR Infant*[TIAB]) AND (“Biological Products”[MeSH] OR Biological Products* [TIAB] OR biologics*[TIAB] OR infliximab*[TIAB] OR adalimumab*[TIAB] OR golimumab*[TIAB] OR certolizumab* [TIAB] OR vedolizumab*[TIAB] OR natalizumab*[TIAB] OR ustekinumab*[TIAB]).
Definitions of clinical outcomes
Biologic exposure: any use of biologic therapy (infliximab, adalimumab, golimumab, certolizumab, natalizumab, vedolizumab, and ustekinumab) from the time of conception to the end of pregnancy. Patients with IBD who stopped using biologics during the third trimester of pregnancy were included. Primary outcome: infantile infections defined as any infection occurring within the first year of life. Secondary outcomes: (i) infantile antibiotic use and (ii) infection-related hospitalizations.
Inclusion and exclusion criteria
Two authors (J.G. and O.H.N.) independently reviewed the abstracts and manuscripts for eligibility. Conflicts were resolved with consultation of another author (C.B.J.). Our inclusion criteria included (i) interventional or observational studies, (ii) pregnant women with IBD with or without biologic exposure, and (iii) reported infantile infections. Our exclusion criteria were (i) case reports, (ii) studies only including patients without exposure to biologic therapy, (iii) no data on infantile infections, and (iv) no control group (pregnancy not exposed to biologics).
Data extraction
The following data were extracted: (i) author names, publication year, and country (or countries) of patient population; (ii) study design; (iii) type of biologic exposures and proportion of mothers with IBD continuing biologics during the third trimester of pregnancy; (iv) maternal IBD type and proportion of patients with active (moderate or severe) disease (defined by individual studies) during pregnancy; (v) proportion of mothers with IBD on steroids during pregnancy; (vi) cohort mean maternal age at the time of pregnancy; (vii) the total number of live births/infants; (viii) the total number of infantile infections; (ix) infections requiring antibiotic use; (x) infection-related hospitalizations; and (xi) the number of acute otitis media (AOM), upper respiratory infection (URI), urinary tract infection (UTI), and gastrointestinal (GI) infection cases.
Assessment of study bias
Two authors (J.G. and O.H.N.) independently assessed the risk of bias in included studies using a modified Newcastle‐Ottawa scale for case‐control studies or cohort studies (19). Significant conflicts between Newcastle‐Ottawa scores were resolved with the consultation of another author (C.B.J.). The following criteria were evaluated: selection, representativeness of cases, definition of controls, comparability (of cases and controls), ascertainment of exposure, and assessment of outcomes. Each domain of the Newcastle‐Ottawa scale was judged for the risk of bias as low, uncertain, or high.
Statistical analyses
Outcomes were extracted from individual manuscripts or calculated using raw data and pooled using a random effects model. Review Manager v5.3 was used to calculate the pooled odds (and 95% confidence interval [CI] and P values) of our clinical outcomes. Heterogeneity was assessed using I2 statistics defined by the Cochrane Handbook for Systematic Reviews (20). We performed a subgroup analysis for the type of infantile infections (AOM, URI, UTI, and GI). Because certolizumab does not cross the placenta and should not affect the risk of infections, we performed a sensitivity analysis comparing the risk of infantile infections in studies including certolizumab vs studies not including this drug. Additional sensitivity analyses included restricting the meta-analysis to studies with only anti-TNF agents, performing the meta-analysis according to the study design (retrospective vs prospective) and risk of bias (low vs high/uncertain). We also performed meta-regression analyses (metareg function, Stata/IC 15.1 for Windows; StataCorp, College Station, TX) to determine whether the proportion of mothers with IBD continuing biologics during the third trimester, on steroids, or with active disease during pregnancy associated with the effect size (Log odds ratio [OR]) of our clinical outcomes. A funnel plot and Egger test were used to assess for publication bias.
RESULTS
Search results
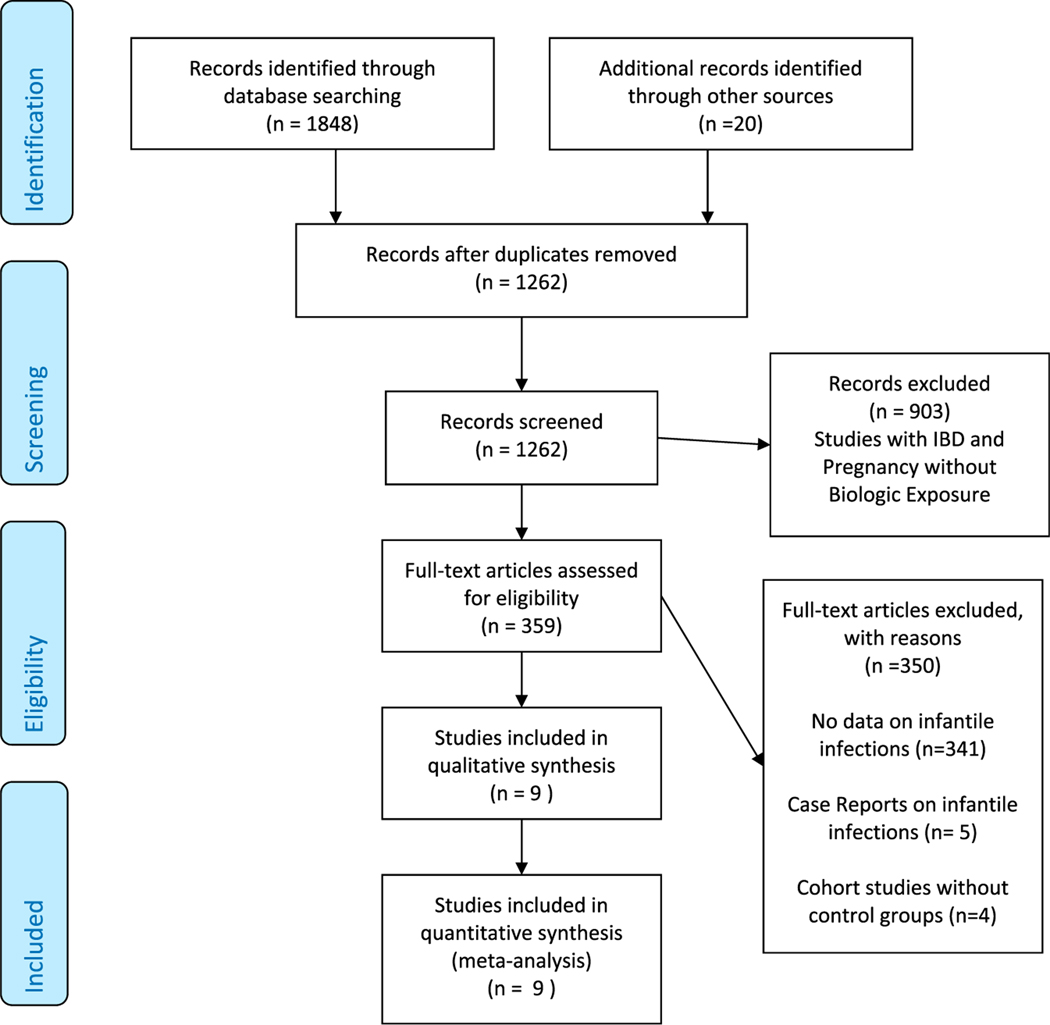
Our systematic review PRISMA flowchart is summarized in Figure 1. After removing duplicates, our search strategy yielded 1,262 citations. A total of 903 studies involving IBD and pregnancy but not biologic therapy were excluded by title and abstract. A total of 359 studies with IBD, pregnancy, and biologic therapy underwent full-text assessment for eligibility. Of these, 350 studies were excluded because they did not report infantile infections, were case reports, or lacked a control group. A total of 9 studies were included for qualitative assessment and meta-analysis.
Figure 1.
PRISMA flowchart—study selection process in the risk of infantile infections with biologic therapy during pregnancy. IBD, inflammatory bowel disease.
Characteristics of included studies
The baseline characteristics of included studies are summarized in Table 1. The 9 included studies (14,21–28) comprised 8,013 women with IBD (5,212 Crohn’s disease, 2,801 ulcerative colitis) who gave birth to 8,490 infants. The mean maternal age at the time pregnancy of was 31 years. 1,965 pregnancies were exposed to biologics, whereas 6,525 pregnancies were not exposed. All included studies were observational. All studies reported infantile infection outcomes with anti-TNF exposure except for 1 study (22), which also reported exposure to vedolizumab and another study which included patients with a mix of biologics including anti-TNF agents and ustekinumab (28). The risk of bias of included studies is summarized in Supplementary Table 1 (see Supplementary Digital Content 2, http://links.lww.com/AJG/B753): 6 studies had low risk of bias (14,21,23–25,27), 1 study was deemed to have uncertain risk of bias (22), and 2 studies had high risk of bias (26,27).
Table 1.
Baseline characteristics of included studies evaluating the risk of infantile infections from pregnancies in IBD women on biologic therapy
| Study | Year | Country | Study design | Biologic therapy | Maternal IBDtype | Maternal age at the time of pregnancy (mean) | Continued biologics during third trimester (proportion) | Total no. of live births | Total infantile infections (≤12 mo old) | Antibiotic use | Infection-related hospitalizations |
|---|---|---|---|---|---|---|---|---|---|---|---|
| de Lima etal. (21) | 2016 | The Netherlands | Prospective | IFX, ADA | 42 CD, 13 UC | 29.9 | 0.30 | 55 | 18 | 4 | 3 |
| Cohort | Control (no anti-TNF) | No data | 31.5 | 459 | 207 | 83 | 3 | ||||
| Moens et al. (22) | 2019 | Multicountry | Retrospective | VDZ | 40 CD, 33 UC | 30 | 0.28 | 64 | 3 | 1 | 3 |
| Case control | IFX, ADA | 136 CD, 28 UC | 30 | 67 | 7 | 3 | 7 | ||||
| Control (no biologic) | 86 CD, 69 UC | 31 | 59 | 7 | 0 | 7 | |||||
| Duricova etal. (23) | 2018 | Czech Republic | Retrospective | IFX, ADA | 52 CD, 20 UC | 35.1 | 0.44 | 72 | 17 | 15 | 17 |
| Case control | Control (no anti-TNF) | No data | No data | 69 | 12 | 12 | 12 | ||||
| Casanova etal. (24) | 2013 | Spain | Retrospective | IFX, ADA, CTZ | 54 CD, 12 UC | 32 | 0.30 | 66 | 2 | 2 | 2 |
| Case control | Control (no anti-TNF) | 116 CD, 202 UC | No data | 318 | 8 | 8 | 8 | ||||
| Seirafi et al. (25) | 2014 | France | Retrospective | IFX, ADA, CTZ | 107 CD, 24 UC | 29.3 | 0.20 | 133 | 2 | 2 | 2 |
| Case control | Control (no anti-TNF) | 71 CD, 24 UC | 28.9 | 99 | 1 | 1 | |||||
| Luu etal. (14) | 2018 | Multicountry | Retrospective | IFX, ADA, CTZ, GOL | 655 CD, 144 UC | 29.4 | 0.68 | 799 | 349 | 161 | 175 |
| Cohort | Control (no anti-TNF) | 3,143 CD, 1,693 UC | 31 | 4,836 | 2,220 | 839 | 908 | ||||
| Soares et al. (26) | 2016 | Portugal | Retrospective | IFX, ADA | 40 CD, 59 UC | 32 | No data | 11 | 4 | No data | 4 |
| Cohort | Control (no anti-TNF) | No data | No data | 24 | 2 | No data | 2 | ||||
| Chaparro etal. (27) | 2018 | Multicountry | Retrospective | IFX, ADA, CTZ | 291 CD, 97 | 31 | 0.38 | 388 | 46 | 15 | 6 |
| Cohort | Control (no anti-TNF) | 190 CD, 263 | 32.5 | 453 | 44 | 9 | 4 | ||||
| Matro etal. (28) | 2018 | USA | Prospective | IFX, ADA, CTZ, GOL, NAT, UST | 189 CD, 121 UC | 31.2 | No data | 310 | 90 | 46 | 31 |
| Cohort | Control (no biologic) | No data | No data | 208 | 94 | 48 | 17 |
ADA, adalimumab; CD, Crohn’s disease; CTZ, certolizumab; GOL, golimumab; IBD, inflammatory bowel disease; IFX, infliximab; NAT, natalizumab; TNF, tumor necrosis factor; UC, ulcerative colitis; UST, ustekinumab; VDZ, vedolizumab.
Risk of all infantile infections
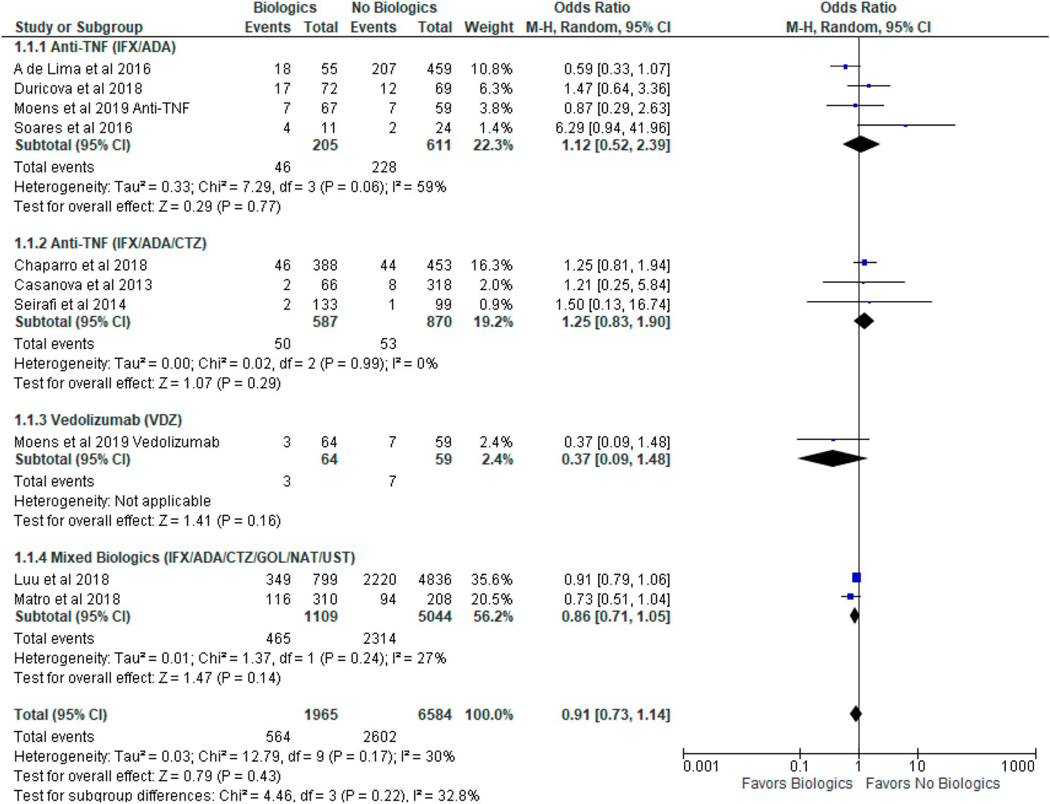
Of the 1,965 pregnancies exposed to biologics, the incidence of all infections was 0.27 cases per infant-year, whereas of the 6,525 pregnancies not exposed to biologics, the incidence was 0.40 cases per infant-year. There were no reported infection-related deaths. We were unable to assess for age of infants at the time of infection because of limited data. Use of biologics in women with IBD during pregnancy was not associated with increased risk of all infantile infections (OR 0.91, 95% CI 0.73–1.14, I2 = 30%) as summarized in Figure 2.
Figure 2.
The risk of subsequent infantile infections after in utero exposure to biologic therapy in women with inflammatory bowel disease. CI, confidence interval.
Subgroup analysis: risk of specific types of major infantile infections
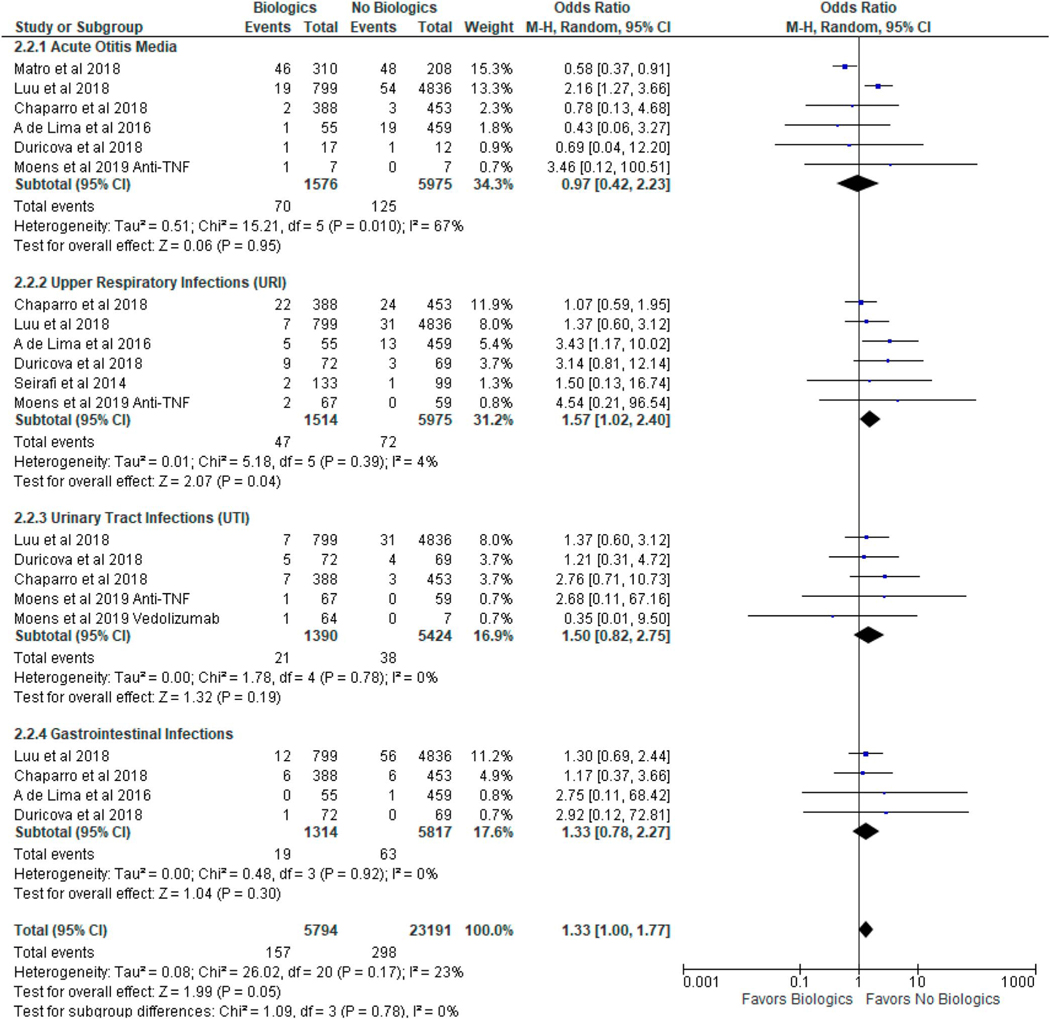
Table 2 summarizes the major types of infantile infections (AOM, URI, UTI, and GI) documented in the included studies. Table 3 summarizes the incidence of major infections from our study compared with meta-analyses of infants in the general population (29–32). In infants exposed to biologics during pregnancy, the pooled incidence (cases per infant-year) of AOM, URI, UTI, and GI were 0.04, 0.02, 0.01, and 0.01, respectively, which were not higher than that of the general population (0.05, 0.18, 0.07, and 0.01, respectively). In infants not exposed to biologics during pregnancy, the pooled incidence (cases per infant-year) of AOM, URI, UTI, and GI were 0.02, 0.01, 0.01, and 0.01, respectively, which were not higher than that of the general population (0.05, 0.18, 0.07, and 0.01, respectively). In a subgroup analysis, biologic use during pregnancy was associated with an increased risk of URIs (OR 1.57, 95% CI 1.02–2.40, I2 = 4%), but not AOM (OR 0.97, 95% CI 0.42–2.23, I2 = 67%), UTIs (OR 1.50, 95% CI 0.82–2.75, I2 = 0%), or GI infections (OR 1.33, 95% CI 0.78–2.27, I2 = 0%) as summarized in Figure 3.
Table 2.
Biologic therapy during pregnancy in women with inflammatory bowel disease and risk of major types of infantile infections
| Study | Year | Biologic therapy | Acute otitis media | Upper respiratory infection infections | Urinary tract infections | Gastrointestinal infections |
|---|---|---|---|---|---|---|
| de Lima etal. (21) | 2016 | IFX, ADA | 1 | 5 | 0 | 0 |
| Control (no anti-TNF) | 19 | 13 | 0 | 1 | ||
| Moens etal. (22) | 2019 | VDZ | 0 | 0 | 1 | 0 |
| IFX, ADA | 1 | 2 | 1 | 0 | ||
| Control (no biologic) | 0 | 0 | 0 | 0 | ||
| Duricova etal. (23) | 2018 | IFX, ADA | 1 | 9 | 5 | 1 |
| Control (no anti-TNF) | 1 | 3 | 4 | 0 | ||
| Casanova etal. (24) | 2013 | IFX, ADA, CTZ | No data | No data | No data | No data |
| Control (no anti-TNF) | No data | No data | No data | No data | ||
| Seirafi etal. (25) | 2014 | IFX, ADA, CTZ | 0 | 2 | 0 | 0 |
| Control (no anti-TNF) | 0 | 1 | 0 | 0 | ||
| Luu et al. (14) | 2018 | IFX, ADA, CTZ, GOL | 19 | 7 | 7 | 12 |
| Control (no anti-TNF) | 54 | 31 | 31 | 56 | ||
| Soares etal. (26) | 2016 | IFX, ADA | No data | No data | No data | No data |
| Control (no anti-TNF) | No data | No data | No data | No data | ||
| Chaparro etal. (27) | 2018 | IFX, ADA, CTZ | 2 | 22 | 7 | 6 |
| Control (no anti-TNF) | 3 | 24 | 3 | 6 | ||
| Matro etal. (28) | 2018 | IFX, ADA, CTZ, GOL, NAT, UST | 46 | No data | No data | No data |
| Control (no anti-TNF) | 48 | No data | No data | No data |
ADA, adalimumab; CTZ, certolizumab; GOL, golimumab; IFX, infliximab; NAT, natalizumab; TNF, tumor necrosis factor; UST, ustekinumab; VDZ, vedolizumab.
Table 3.
Incidence of infections in infants in the general population versus infants born to mothers with inflammatory bowel disease (IBD) with or without biologics during pregnancy
| Pooled incidence of infection (cases per infant-year) |
||||
|---|---|---|---|---|
| Infants in general population |
Infants born to IBD mothers |
|||
| Infantile infections | Pooled incidence | Study details | Biologic pregnancy exposure | No biologic pregnancy exposure |
| Acute otitis media | 0.05 | Monasta etal. (29), meta-analysis | 0.04 | 0.02 |
| 35.7 million cases, 788 million Infant-year | Global acute otitis media | 70 cases, 1,965 infant-year | 125 cases, 6,525 infant-year | |
| Upper respiratory infections | 0.18 | Shi etal. (30), meta-analysis | 0.02 | 0.01 |
| 14, 898 cases, 83,220 infant-year | Global viral respiratory infections | 47 cases, 1,965 infant-year | 72 cases, 6,525 infant-year | |
| Urinary tract infections | 0.07 | Shaikh etal. (31), meta-analysis | 0.01 | 0.01 |
| 1,159 cases, 17,421 infant-year | Urinary tract infections | 21 cases, 1,965 infant-year | 38 cases, 6,525 infant-year | |
| Gastrointestinal infections | 0.01 | Ahmed etal. (32), meta-analysis | 0.01 | 0.01 |
| 431 cases, 69,040 infant-year | Global gastroenteritis | 19 cases, 1,965 infant-year | 63 cases, 6,525 infant-year | |
Figure 3.
Subgroup analysis of specific types of infantile infections after in utero exposure to biologic therapy in women with inflammatory bowel disease. CI, confidence interval.
Risk of antibiotic use and infection-related hospitalizations
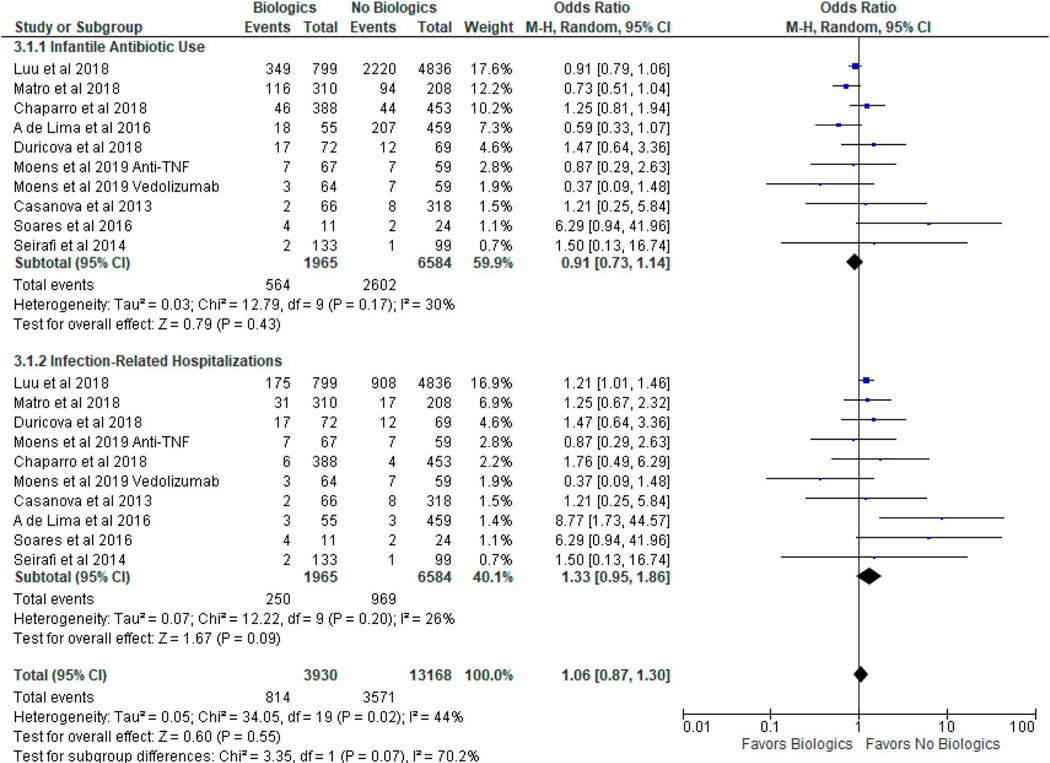
Eight studies (14,21–25,27,28) reported the rates of infantile antibiotic use, whereas all 9 studies (14,21–28) reported the rates of infection-related hospitalizations. In infants exposed to biologics during pregnancy, the incidence (cases per infant-year) of infections requiring antibiotics and infection-related hospitalizations were 0.13 and 0.13, respectively. In infants not exposed to biologics during pregnancy, the incidence (cases per infant-year) of infections requiring antibiotics and infection-related hospitalizations were 0.15 and 0.15, respectively. Biologic use in pregnant women with IBD was not associated with increased risk of infantile antibiotic use (OR 0.91, 95% CI 0.73–1.14, I2 = 30%) or increased risk of infection-related hospitalizations (OR 1.33, 95% CI 0.95–1.86, I2 = 26%) as summarized in Figure 4.
Figure 4.
The risk of infantile antibiotic use and infection-related hospitalizations after in utero exposure to biologic therapy in women with inflammatory bowel disease. CI, confidence interval.
Publication bias and sensitivity analyses
A funnel plot (see Supplementary Figure 1, Supplementary Digital Content 1, http://links.lww.com/AJG/B752) of included studies showed no evidence of publication bias. An Egger test did not suggest publication bias (P = 0.57). In a sensitivity analysis restricted to only anti-TNF studies (see Supplementary Figure 2, Supplementary Digital Content 1, http://links.lww.com/AJG/B752), biologics during pregnancy in women with IBD was not associated with the risk of infantile infections (OR 1.00, 95% CI 0.77–1.29, I2 = 25%). There was no association between biologics in pregnancy and infantile infections in meta-analyses (see Supplementary Figure 3, Supplementary Digital Content 1, http://links.lww.com/AJG/B752) including studies without certolizumab (OR 1.12, 95% CI 0.52–2.39, I2 = 59%) or with certolizumab (OR 0.91, 95% CI 0.80–1.04, I2 = 0%). The meta-analysis (see Supplementary Figure 4, Supplementary Digital Content 1, http://links.lww.com/AJG/B752) stratified by study design (retrospective vs prospective) revealed significant differences (P = 0.04) in the risk of infantile infections. In prospective studies (2 studies), biologic exposure during pregnancy in women with IBD was associated with the decreased risk of infantile infections (OR 0.69, 95% CI 0.51–0.93, I2 = 0%). By contrast, in retrospective studies (7 studies), there was no association (OR 1.04, 95% CI 0.80–1.35, I2 = 19%) between biologic exposure and infantile infections. The meta-analysis stratified by the risk of bias (low vs uncertain/high bias) (see Supplementary Figure 5, Supplementary Digital Content 1, http://links.lww.com/AJG/B752) revealed no significant differences in the risk of infantile infections. Meta-regression analyses revealed that the proportion of mothers with IBD continuing biologics during the third trimester (Figure 5) on steroids during pregnancy (see Supplementary Figure 6, Supplementary Digital Content 1, http://links.lww.com/AJG/B752) or with active disease during pregnancy (see Supplementary Figure 7, Supplementary Digital Content 1, http://links.lww.com/AJG/B752) were not associated with the risk of all infantile infections, 4 major infantile infections (AOM, URI, UTI, and GI), antibiotic use, or infected-related hospitalizations.
Figure 5.
Meta-regression analyses of the proportion of inflammatory bowel disease mothers continuing biologics during the third trimester and odds of (a) all infantile infections, (b) acute otitis media, (c) upper respiratory infections, (d) urinary tract infections, (e) gastrointestinal infections, (f) infantile antibiotic use, and (g) infection-related hospitalizations. CI, confidence interval; OR, odds ratio.
DISCUSSION
To our knowledge, this is the first meta-analysis quantifying the risk of infantile infections after in utero biologic exposure as part of IBD therapy in pregnancy. In this systematic review and meta-analysis comprising over 8,000 infants, we demonstrate that biologic use is not associated with an increased risk of all infantile infections. Although we observed an increased risk of URIs in the subgroup analysis, biologic use during pregnancy was not associated with an increased risk of serious infections requiring antibiotics or hospitalizations. There was no reported infection-related infant mortality. We also show that the risk of infantile infection is comparable between certolizumab vs other TNF inhibitors and that continuing biologics during the third trimester does not seem to confer an increased infection risk.
The incidence of major infections in infants born to mothers with IBD with or without biologic exposure in our meta-analysis did not seem to be increased compared with infants in the general population. In a meta-analysis of 114 studies (29), the pooled global incidence of AOM was 0.05 cases per infant-year, which was comparable with our results. In another meta-analysis (30), the global incidence of viral respiratory infections was 0.18, which was much higher than our incidence of URIs in infants with or without biologic exposure during pregnancy. The incidence of UTI and GI infections in infants in our study were not higher than that reported in previous meta-analyses (31,32) of infants in the general population.
Our finding that the use of biologics during pregnancy in women with IBD is not associated with an increased risk of infantile infections could have several explanations. First, although biologics undergo transplacental transfer and persist at detectable drug concentrations in infantile circulation, it is possible that any immunocompromising effects are transient and changes in immune function normalize once the drug is cleared. Indeed, this is supported by a previous prospective study (33) which showed that infants exposed to TNF inhibitors in utero had detectable concentrations of anti-TNF at birth and a more immature B and helper-T phenotype and decreased regulatory T cell frequency. These immune changes normalized after anti-TNF levels became undetectable at 6 months of age. None of the infants experienced any infections. This idea is further supported by recent data demonstrating that biologic use by pregnant women with IBD does not affect infant response to routine (nonlive) vaccines at 2–6 months of age, which are routinely given in the first year of life (34). Second, it is possible that biologic exposure does cause some immunocompromise in the infant but that passive immunity from maternal transfer of cytokines and protective antibodies through the placenta and breastmilk (35–37) abrogates this effect. Transfer of biologics through breastmilk have been reported tobe very low (28). Third, an alternative explanation is that blockade of pathways by biologics may not be critical for common infections in infants or infections that are affected by biologic blockade (e.g., tuberculosis) were not endemic in the included patient populations and not captured by our analysis.
We observed that biologic therapy during pregnancy was associated with an increased risk of infantile URIs. The only other study adequately powered to detect this subtle risk did not quantify URIs in infants born to mothers treated with biologics (29). We hypothesize that this association may have been missed by other studies because the effect is subtle, and all of the infections were self-limited and not associated with an increased risk of hospitalization. The self-limited alterations in the immune cell repertoire of infants exposed in utero tobiologics (33) may cause a mildly immunocompromised state reflected in an increase in URI frequency, without compromising vaccine efficacy or predisposing to serious infections requiring hospitalization.
Our study has several strengths. First, we conducted a meta-analysis of multiple large populations, increasing the statistical power to detect a subtle association between biologic use during pregnancy and infantile infections and to resolve conflicting data and uncertainty from previous studies. Second, we included diverse cohorts of pregnant women with IBD from different countries to overcome geographic and institutional bias broadening the generalizability of our findings. Third, the clinical outcomes we examined were comprehensive including the risk of all infantile infections and more clinically meaningful end points such as infantile antibiotic use and infection-related hospitalizations while being granular enough to detect subtle immune derangements that may result from biologic exposure. Fourth, heterogeneity and risk of bias of included studies was mostly low. Finally, we performed sensitivity analyses including meta-regression analyses to assess potential confounders for the association between biologics and infantile infection, such as maternal use of biologics during the third trimester, steroid use, and disease activity with our results remaining robustly consistent with our central conclusion. Our study has, however, some limitations. First, we performed meta-analyses of observational studies and thus cannot establish causality. Nevertheless, some factors in our study may support causality such as temporality (biologic exposure in pregnancy preceding outcome of infantile infection) and biologic plausibility (we provided possible mechanisms to explain why biologic therapy during pregnancy may not affect the risk of infection). Performing interventional studies to assess the impact of biologic therapy during pregnancy on the risk of infantile infections poses serious ethical dilemmas. Second, some of our pooled Ors were unadjusted, thus we were unable to adjust for unmeasured confounders such as maternal comorbidities, infant age, and concurrent thiopurine use. Third, our results predominantly reflect the impact of TNF inhibitors because our meta-analyses included only 1 study with vedolizumab and 1 mixed study with ustekinumab.
In conclusion, we provide reassuring evidence that biologic therapy in pregnant women with IBD is not associated with increased risk of infantile infections or serious infections requiring antibiotics or hospitalizations, although biologics may be associated with a subtle URI risk. We show that the risk of infantile infections in certolizumab is comparable with other anti-TNF agents, suggesting that avoiding more efficacious anti-TNF therapy in pregnant women with IBD may not be warranted. Finally, we demonstrate that continuing biologics during the third trimester does not confer additional infection risk. Our study addresses critical questions raised by patients and clinicians and reinforces that the benefits of continuing biologic therapy throughout pregnancy to maintain disease remission outweighs the risks of infantile infections.
Supplementary Material
Study Highlights.
WHAT IS KNOWN
✔ Biologic use in patients with inflammatory bowel disease is associated with an increased risk of infections.
✔ Biologics can cross the placenta during pregnancy and persist at detectable concentrations in infants.
✔ Maternal transfer of biologics during pregnancy may affect infant immune development.
✔ The risk of infections in infants exposed to biologics in utero is controversial.
WHAT IS NEW HERE
✔ Biologics during pregnancy is not associated with an increased risk of all infantile infections.
✔ The risk of upper respiratory infections may be higher in infants exposed to biologics during pregnancy.
✔ Biologics during pregnancy is not associated with the risk of infantile antibiotic use or infection-related hospitalizations.
✔ Therisk of infantile infections was not different between exposure to certolizumab vs other antitumor necrosis factor agents.
Acknowledgments
Financial support: J.G. is in part supported by a Chan Zuckerberg Biohub Physician Scientist Scholar Award and an NIH NIDDK LRP Award.
Guarantor of the article: John Gubatan, MD.
Specific author contributions: J.G. and O.H.N. planned and designed the study and analyzed the data. J.G. and O.H.N. performed the systematic review and extracted the data from manuscripts. J.G. and O.H.N. performed the quality assessment of studies. J.G. performed the statistical analyses in collaboration with C.B.J. S.L. assisted with background literature review and manuscript drafting and provided clinical insight regarding pediatric infections. O.H.N., C.M., S.E.S., C.B.J., and A.H. provided critical review of the manuscript. J.G. drafted the manuscript. All authors interpreted the results and contributed to the critical review of the manuscript. J.G. had full access to the study data and takes responsibility for the integrity of the data and accuracy of the analysis.
Financial support: J.G. is in part supported by a Chan Zuckerberg Biohub Physician Scientist Scholar Award and an NIH NIDDK LRP Award.
Footnotes
CONFLICTS OF INTEREST
Potential competing interests: None to report.
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/B752;http://links.lww.com/AJG/B753
REFERENCES
- 1.Kaplan GG. The global burden of IBD: From 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015;12(12):720–7. [DOI] [PubMed] [Google Scholar]
- 2.Beaulieu DB, Kane S. Inflammatory bowel disease in pregnancy. Gastroenterol Clin 2011;40(2):399–413. [DOI] [PubMed] [Google Scholar]
- 3.McConnell RA, Mahadevan U. Pregnancy and the patient with inflammatory bowel disease: Fertility, treatment, delivery, and complications. Gastroenterol Clin 2016;45(2):285–301. [DOI] [PubMed] [Google Scholar]
- 4.Boyd HA, Basit S, Harpsøe MC, et al. Inflammatory bowel disease and risk of adverse pregnancy outcomes. PLoS One 2015;10(6):e0129567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen OH, Ainsworth MA. Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med 2013;369(8):754–62. [DOI] [PubMed] [Google Scholar]
- 6.Shelton E, Allegretti JR, Stevens B, et al. Efficacy of vedolizumab as induction therapy in refractory IBD patients: A multicenter cohort. Inflamm Bowel Dis 2015;21(12):2879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickson I. IBD: Ustekinumab therapy for Crohn’s disease. Nat Rev Gastroenterol Hepatol 2016;14(1):4. [DOI] [PubMed] [Google Scholar]
- 8.Yu H, MacIsaac D, Wong JJ, et al. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment Pharmacol Ther 2018;47(3):364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borman ZA, Côté-Daigneault J, Colombel JF. The risk for opportunistic infections in inflammatory bowel disease with biologics: An update. Expert Rev Gastroenterol Hepatol 2018;12(11):1101–8. [DOI] [PubMed] [Google Scholar]
- 10.Ford AC, Peyrin-Biroulet L. Opportunistic infections with anti-tumor necrosis factor-α therapy in inflammatory bowel disease: Meta-analysis of randomized controlled trials. Am J Gastroenterol 2013;108(8):1268–76. [DOI] [PubMed] [Google Scholar]
- 11.Bonovas S, Fiorino G, Allocca M, et al. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: A systematic review and network meta-analysis. Clin Gastroenterol Hepatol 2016;14(10):1385–97. [DOI] [PubMed] [Google Scholar]
- 12.Julsgaard M, Christensen LA, Gibson PR, et al. Concentrations of adalimumab and infliximab in mothers and newborns, and effects on infection. Gastroenterology 2016;151(1):110–9. [DOI] [PubMed] [Google Scholar]
- 13.Cheent K, Nolan J, Shariq S, et al. Case report: Fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis 2010;4(5):603–5. [DOI] [PubMed] [Google Scholar]
- 14.Luu M, Benzenine E, Doret M, et al. Continuous anti-TNFα use throughout pregnancy: Possible complications for the mother but not for the fetus. A retrospective cohort on the French National Health Insurance Database (EVASION). Am J Gastroenterol 2018;113(11):1669–77. [DOI] [PubMed] [Google Scholar]
- 15.Mariette X, Förger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: Results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis 2018;77(2):228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hazlewood GS, Rezaie A, Borman M, et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn’s disease: A network meta-analysis. Gastroenterology 2015;148(2):344–54. [DOI] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000;283(15): 2008–12. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med 2009;151(4):264–9. [DOI] [PubMed] [Google Scholar]
- 19.Bent S, Padula A, Avins A. Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. Brief communication: Better ways to question patients about adverse medical events: A randomized, controlled trial. Ann Intern Med 2006;144(4):257–61. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thomas J, Chandler J, et al. (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019). Cochrane, London, 2019. (www.training.cochrane.org/handbook). Accessed October 11, 2019. [Google Scholar]
- 21.de Lima A, Zelinkova Z, van der Ent C, et al. Tailored anti-TNF therapyduring pregnancy in patients with IBD: Maternal and fetal safety. Gut 2016;65(8):1261–8. [DOI] [PubMed] [Google Scholar]
- 22.Moens A, van der Woude CJ, Julsgaard M, et al. Pregnancy outcomes ininflammatory bowel disease patients treated with vedolizumab, anti-TNF or conventional therapy: Results of the European CONCEIVE study. Aliment Pharmacol Ther 2020;51(1):129–38. [DOI] [PubMed] [Google Scholar]
- 23.Duricova D, Dvorakova E, Hradsky O, et al. Safety of anti-TNF-alpha therapy during pregnancy on long-term outcome of exposed children: A controlled, multicenter observation. Inflamm Bowel Dis 2018;25(4):789–96. [DOI] [PubMed] [Google Scholar]
- 24.Casanova MJ, Chaparro M, Domenech E, et al. Safety of thiopurines andanti-TNF-α drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol 2013;108(3):433. [DOI] [PubMed] [Google Scholar]
- 25.Seirafi M, De Vroey B, Amiot A, et al. Factors associated with pregnancy outcome in anti-TNF treated women with inflammatory bowel disease. Aliment Pharmacol Ther 2014;40(4):363–73. [DOI] [PubMed] [Google Scholar]
- 26.Soares E, Gravito-Soares M, Mendes S, et al. Biologic therapy and pregnancy in inflammatory bowel disease: The long-term safety of in utero exposure in a tertiary center [Conference Abstract]. United Eur Gastroenterol J 2016;4(5S):P1418. [Google Scholar]
- 27.Chaparro M, Verreth A, Lobaton T, et al. Long-term safety of in utero exposure to anti-TNFα drugs for the treatment of inflammatory bowel disease: Results from the multicenter European TEDDY Study. Am J Gastroenterol 2018;113(3):396–403. [DOI] [PubMed] [Google Scholar]
- 28.Matro R, Martin CF, Wolf D, et al. Exposure concentrations of infants breastfed by women receiving biologic therapies for inflammatory bowel diseases and effects of breastfeeding on infections and development. Gastroenterology 2018;155(3):696–704. [DOI] [PubMed] [Google Scholar]
- 29.Monasta L, Ronfani L, Marchetti F, et al. Burden of disease caused by otitis media: Systematic review and global estimates. PLoS One 2012;7(4):e36226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi T, McLean K, Campbell H, et al. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: A systematic review and meta–analysis. J Glob Health 2015;5(1):010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaikh N, Morone NE, Bost JE, et al. Prevalence of urinary tract infection in childhood: A meta-analysis. Pediatr Infect Dis J 2008;27(4):302–8. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed SM, Hall AJ, Robinson AE, et al. Global prevalenceof norovirus in cases of gastroenteritis: A systematic review and meta-analysis. Lancet Infect Dis 2014;14(8):725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esteve-Solé A, Deyà-Martínez A, Teixidó I, et al. Immunological changes in blood of newborns exposed to anti-TNF-α during pregnancy. Front Immunol 2017;8:1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beaulieu DB, Ananthakrishnan AN, Martin C, et al. Use of biologic therapy by pregnant women with inflammatory bowel disease does not affect infant response to vaccines. Clin Gastroenterol Hepatol 2018;16(1): 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaretsky MV, Alexander JM, Byrd W, et al. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol 2004;103(3):546–50. [DOI] [PubMed] [Google Scholar]
- 36.Eglinton BA, Roberton DM, Cummins AG. Phenotype of T cells, their soluble receptor levels, and cytokine profile of human breast milk. Immunol Cel Biol 1994;72(4):306–13. [DOI] [PubMed] [Google Scholar]
- 37.Shahid NS, Steinhoff MC, Roy E, et al. Placental and breast transfer of antibodies after maternal immunization with polysaccharide meningococcal vaccine: A randomized, controlled evaluation. Vaccine 2002;20(17–18):2404–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.