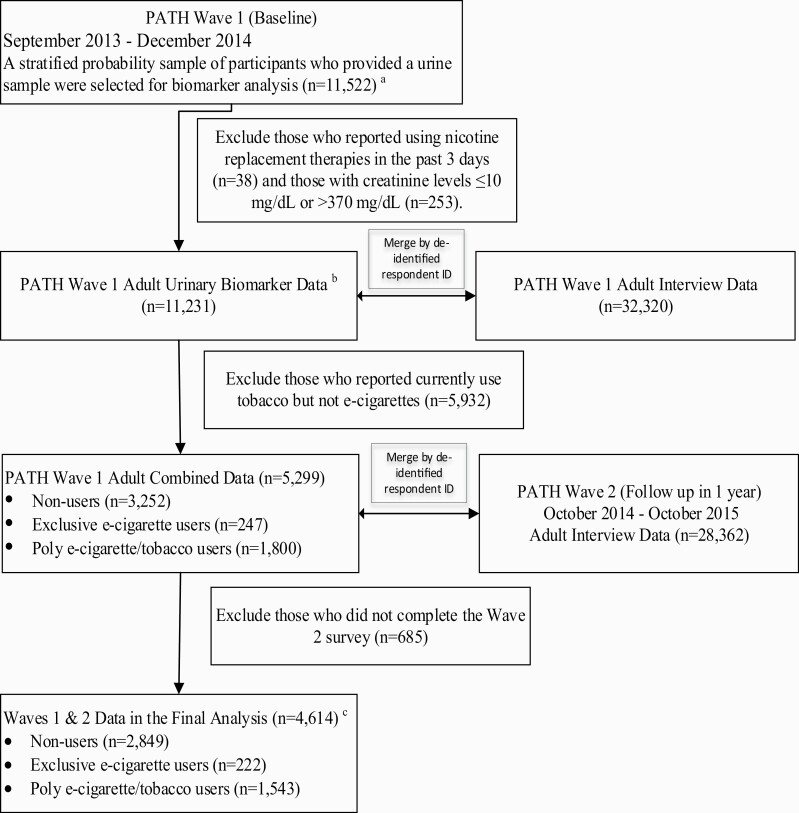
Figure 1.
A flow chart of longitudinal study design and subject selection criteria. aWave 1 final person-level urinary specimen sampling weight and 100 replicate weights were applied to produce a nationally representative dataset of U.S. adults with varying tobacco use statuses at Wave 1. bRespondents reported their use of all nicotine-containing products during a 3-day period prior to the time of any biospecimen collection (Nicotine Exposure Questions [NEQs]). NEQs were incorporated into the adult interview. cThe results of the final analysis are presented in the main text. A sensitivity analysis was performed by excluding subjects with a history of respiratory diseases, including COPD, chronic bronchitis, emphysema, asthma, and some other lung or respiratory condition at Wave 1. See Supplementary material.