Fig. 4. pH dependence of KirBac1.1 activity.
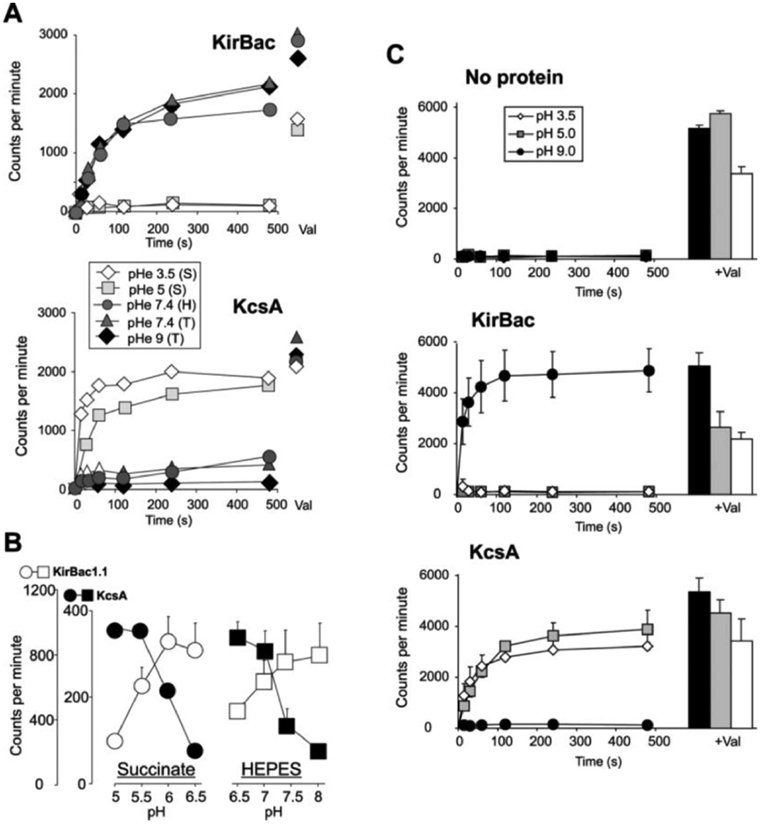
A, time course of 86Rb+ uptake in liposomes reconstituted with KirBac1.1 or KcsA. External pH (pHe) was adjusted to 3.5–9 using succinate (S), HEPES (H), or Tris (T) buffers as indicated. Internal pH was originally buffered to pH 7.0 with HEPES. B, 86Rb+ uptake at 8 min as a function of pHe for KcsA (filled, symbols) and KirBac1.1 (open symbols), using succinate (left)- or HEPES (right)-buffered solutions. Internal liposomal pH was 7.0 with HEPES throughout (n = 3, mean ± S.E.). C, time course of 86Rb+ uptake in liposomes reconstituted with no protein, KirBac1.1, or KcsA. pHe was adjusted to 3.5, 5 (using succinate), or 9 (using Tris. Internal pH was originally buffered to 7.0 with HEPES. To the right are mean (+S.E.) uptakes after addition of valinomycin.
