Abstract
Background:
Lung cancer (LC) is a common malignancy and leading cause of cancer death worldwide and in Thailand. An update on LC survival factors after diagnosis at Srinagarind Hospital is needed.
Methods:
We conducted a retrospective cohort study, and the data were sourced from the Srinagarind Hospital-Based Cancer Registry. All LC cases were diagnosed between January 1, 2013, and December 31, 2017, and followed up until November 30, 2019. Cases of LC (ICD-O-3) numbered 2,149, but only those with coding C34.0-C34.9 were included. The survival rate was estimated using Kaplan-Meier, while the Log-rank test was used to estimate survival. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox proportional hazard regression models.
Results:
The 2,149 patients had a total follow-up of 269.6 person-years. Overall, 1,867 patients died during the study, for a corresponding case-fatality mortality rate of 86.0 per 100 person-years. The respective 1-, 3-, and 5-year survival rate was 31.2 % (95% CI; 29.21 to 33.15%), 12.9 % (95%CI: 11.49 to 14.45), and 10.2% (95%CI: 8.74 to 11.70). After patient diagnosis, the median survival time was 0.46 years (5.51 months) (95% CI: 0.42 to 0.50). Targeted therapy was associated with longer survival than non-targeted therapy (p-value < 0.001). After adjusting for sex, TNM stage, and histologic type, multivariable analysis of the entire cohort identified chemotherapy as an independent predictor of improved survival (adjusted HR= 0.48; 95% CI: 0.42 to 0.55; P < 0.001), and that sex, TNM stage, and histologic type were associated with survival.
Conclusion:
The study confirmed that sex, stage of disease, histology, and chemotherapy are associated with survival of LC. Primary prevention and screening for early detection improve survival. Further investigations into factors affecting survival of LC in Northeast Thailand should focus on targeted therapy.
Key Words: Lung cancer, survival, cancer registry, targeted therapy
Introduction
Lung cancer (LC) is a malignant tumor with the highest global morbidity and mortality of all cancers. In 2018, the number of new cases was 2,093,876 (11.6% of all cancers), while the number of deaths was 1,761,007 (18.4% of all cancers) among both sexes for all ages (Bray et al., 2017). The respective age-standardized rate (ASR) for LC in males and females is 31.5 and 14.6 per 100,000. The respective age-standardized incidence and mortality rate for both sexes is 22.5 and 18.6 per 100,000 (International Agency for Research on Cancer, 2019).
For Thais, the ASR for LC is between 20.6 and 27.1 per 100,000 in males and between 9.3 and 11.9 per 100,000 in females. In Khon Kaen province, Thailand, the ASR is between 18.0 and 21.1 per 100,000 in males and between 5.9 and 7.6 per 100,000 in females. The most common histological type is non-small cell lung cancer (NSCLC) (90.0%), followed by small cell lung cancer (SCLC) (10.0%) (Sriplung et al., 2003; Khuhaprema et al., 2007; Khuhaprema et al., 2010; Khuhaprema et al., 2012).
The incidence trend for LC in Khon Kaen province, Thailand, has modestly increased over the last 20 years. By 2030, the incidence rate is predicted to increase among females but to decline among males (Santong et al., 2018). Prevention is a long-term strategy, and not all cancers can be prevented (Schottenfeld and Fraumeni, 2006); nevertheless, implementation of national and regional preventative programming has been uneven. In order to reduce LC mortality, a reduction of LC incidence and an improvement of LC survival are essential (Allemani et al., 2018). The first-ever population-based cancer survival data (1985-1992) for Khon Kaen, Thailand, were published in 1995. The study revealed that the most common cancers in the province were liver (5-year relative survival rate 9.2%), cervix (60.1%), lung (15.4%), breast (48.1%), and large bowel (41.9%) (Sriamporn et al., 1995). A later study on Cancer Survival in Khon Kaen, Thailand, revealed that the 5-year survival between 1993 and 1997 was highest for localized disease, followed by regional and distant metastatic categories. Trends in the 5-year relative survival between 1993 and 1997 vs. 1985 and 1992 showed a marked increase for cancers of the rectum, breast, ovary, Hodgkin and non-Hodgkin lymphomas, and a decrease for cancers of the lip and larynx (Suwanrungruang et al., 2011). Previous studies showed the prognostic factors in non-small cell LC patients include stage of disease, performance status (León-Atance et al., 2011), weight loss, male vs. female, age, smoking status, smoking history, quality of life, marital status, diagnosed with depression, and genetic mutations (Jazieh et al., 2000; Brundage et al., 2002).
The prognostic factors and survival rate for LC have not been updated recently for the tertiary hospitals in northeastern Thailand where cancer patients are treated. The current research thus aimed to determine the factors affecting the survival of LC patients after diagnosis at Srinagarind Hospital.
Materials and Methods
Cancer Registries and Case Ascertainment
Khon Kean Cancer Registry, KKCR
The Khon Kaen Cancer Registry (KKCR) was established in 1984 at the Faculty of Medicine and Srinagarind Hospital, Khon Kaen University, Khon Kaen, Thailand. It comprises both hospital and population-based registrations. The KKCR contains data on 1.7 million patients comprising all cancer sites as per the International Agency for Research on Cancer (IARC) guidelines (Esteban et al., 1995).
Case definitions
The database was retrieved for all patients with LC tumors treated at Srinagarind Hospital, Faculty of Medicine, Khon Kaen University between January 1, 2013, and December 31, 2017. Diagnoses were obtained using the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3). LC is an ICD-O-3 diagnosis and only includes coding C34.0-C34.9 (World Health Organization, 2013).
Statistical methods
Descriptive epidemiology of study patients
The characteristics of the patients were summarized using descriptive statistics. Means and standard deviations, medians, and their ranges (minima and maxima) were used for continuous variables, and frequency counts and percentages were used for categorical variables.
Survival analyses
Survival analyses excluded cases if their basis of diagnosis was Death Certificate Only (DCO) or unknown, if they did not contain any follow-up information, or had an unknown vital status. Survival was determined by calculating the follow-up time from diagnosis to each patient’s last known vital status. The status was obtained by linking records between the Mortality Registry of Thailand (National Health Office, 2017) and the National Statistical Office (National Statistical Office Thailand, 2017, updated to December 31, 2016).
The observed survival (OS) analysis was estimated using the Kaplan-Meier survival curve, and the log-rank test was used for between-group comparisons. Multivariable analysis was performed using Cox proportional hazards regression (Kleinbaum and Klein, 2005). All test statistics were two-sided, and a p-value of < 0.05 was considered statistically significant.
Data processing
Data were recorded using the CanReg 5 software provided by the International Association of Cancer Registries (IARC) (IARC, 2019). The verification was performed with necessary corrections, including logic, range, and internal consistency, which were checked using statistical software. All analyses were performed using Stata release 10.0 (StataCorp LLC, College Station, TX, USA). (Stata Corp, 2007)
Ethical considerations
This project was reviewed and approved by the Human Research and Ethics Committee of Khon Kaen University (HE631214).
Results
Descriptive epidemiology and Data quality
Between 2013 and 2017, 2,149 cases of LC were recorded in the Srinagarind Hospital-Based Cancer Registry database. Male LC patients outnumbered female LC patients. The age at diagnosis trended to be late middle-aged (mean, 62.4 years; standard deviation, 11.3; median, 63.0 years; Min: Max; 20: 91). Most were “married” (n=2,041; 95.1%). As for the year of diagnosis, the most numerous was in 2014 (n=497; 23.1%), while the least was in 2017 (n=364; 16.9%).
The basis of diagnosis was endoscopic and radiologic evidence vs. morphological verification (n=1,534; 71.4%) (i.e., based on either cytological or histological examination of tissue from the primary site, %MV). Based on the subtype of cancer, the highest was in the upper lobe (n=818; 38.1%), while the lowest was overlapping lesions of the lung (n=35; 1.6%). The most common histological grading was adenocarcinoma (n=930; 43.3%), while the highest was “unknown grading” (n=1,815; 84.5%).
The most common stage of diseases were ‘Stage IV’ (n=1,150; 53.5% and “Stage I” (n=35; 1.6%). A histological grading was commonly lacking (males; n=9,199, 97.6%; females; n=4,282, 97.9%). Tumour laterality was often unknown or undefined (n=885, 41.2%). Metastatic lung cancer was bone metastasis (n=288, 13.4%). For treatment, only targeted therapy was offered to 37 patients (1.7%) while supportive care was offered to 1,198 (55.8%) (Table 1).
Table 1.
Characteristics of Study Participants Diagnosed at Srinagarind Hospital between 2013 and 2017
| Characteristic | Number (n = 2,149) |
Percentage (%) |
|---|---|---|
| Sex | ||
| Male | 1,420 | 66.1 |
| Female | 729 | 33.9 |
| Age at diagnosis (years) | ||
| 20-29 | 14 | 0.7 |
| 30-39 | 47 | 2.2 |
| 40-49 | 205 | 9.5 |
| 50-59 | 571 | 26.6 |
| 60-69 | 718 | 33.4 |
| 70-79 | 485 | 22.6 |
| >80 | 109 | 5.1 |
| Mean (standard deviation) | 62.4 (11.3) | |
| Median (minimum: maximum) | 63.0 (20: 91) | |
| Marital status | ||
| Single | 59 | 2.8 |
| Married | 2041 | 95.1 |
| Monk | 42 | 2 |
| Not specify | 7 | 0.3 |
| Year of Diagnosis | ||
| 2013 | 489 | 22.8 |
| 2014 | 497 | 23.1 |
| 2015 | 385 | 17.9 |
| 2016 | 414 | 19.3 |
| 2017 | 364 | 16.9 |
| Basis of diagnosis | ||
| History & Physical exam | 5 | 0.2 |
| Ultrasound (Endoscopy & Radiology) | 602 | 28 |
| Surgery & Autopsy (no histol.) | 6 | 0.3 |
| Specific Biochem/ Immuno. test | 2 | 0.1 |
| Cytology or Hematology | 195 | 9.1 |
| Histology of Metastasis | 24 | 1.1 |
| Histology of Primary | 1315 | 61.2 |
| Subtype | ||
| Main bronchus | 51 | 2.4 |
| Upper lobe, lung | 818 | 38.1 |
| Middle lobe, lung | 82 | 3.8 |
| Lower lobe, lung | 466 | 21.7 |
| Overlapping lesion of lung | 35 | 1.6 |
| Lung, NOS | 697 | 32.4 |
| Histology | ||
| Squamous cell carcinoma | 156 | 7.3 |
| Adenocarcinoma | 930 | 43.3 |
| Small cell carcinoma | 43 | 2 |
| Large cell carcinoma | 160 | 7.5 |
| Other specified carcinoma | 34 | 1.6 |
| Sarcoma | 8 | 0.4 |
| Non-small cell carcinoma | 128 | 6 |
| Other specified malignant neoplasm | 9 | 0.4 |
| Unspecified malignant neoplasm | 681 | 31.7 |
| Characteristic | Number (n = 2,149) |
Percentage (%) |
| Histology grading | ||
| Well differentiated | 50 | 2.3 |
| Moderately differentiated | 56 | 2.6 |
| Poorly differentiated | 196 | 9.1 |
| Undifferentiated | 32 | 1.5 |
| Not known | 1815 | 84.5 |
| Laterality | ||
| Right | 689 | 32.1 |
| Left | 541 | 25.2 |
| Bilateral | 34 | 1.6 |
| Unknown | 885 | 41.2 |
| Stage of disease | ||
| Stage I | 35 | 1.6 |
| Stage II | 49 | 2.3 |
| Stage III | 356 | 16.6 |
| Stage IV | 1150 | 53.5 |
| Unknown | 559 | 26 |
| Metastasis | ||
| Lymp node metastasis | 70 | 3.3 |
| Bone metastasis | 288 | 13.4 |
| Liver metastasis | 79 | 3.7 |
| Treatment | ||
| Surgery | 140 | 6.5 |
| Radiation | 393 | 18.3 |
| Chemotherapy | 669 | 31.1 |
| Targeted therapy | 37 | 1.7 |
| Supportive care | 910 | 42.4 |
Survival rate of LC after diagnosis
Mortality rate and Median survival time
The 2,149 patients had a total follow-up of 269.6 person-years. Overall, 1,867 patients died during the study, corresponding to a mortality rate (case-fatality) of 86.0 per 100 person-years. The respective 1-, 3-, and 5-year survival rate was 31.2 % (95% confidence interval; 29.21 to 33.15%), 12.9 % (95%CI: 11.49 to 14.45), and 10.2% (95%CI: 8.74 to 11.70). After diagnosis, the median survival time was 0.46 years (5.51 months) (95% CI: 0.42-0.50).
Based on the type of cancer, the respective median overall survival (OS) and 3-year OS rates for patients with small-cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) was 0.58 year (95%CI: 0.39 - 0.76) and 11.6% (95%CI: 4.26 to 23.06) vs. 0.60 years (95%CI: 0.53 to 0.66) and 15.1% (95%CI: 13.21 to 17.19). As for SCLC, the respective 1-year OS rate for patients treated with chemotherapy vs. non-chemotherapy was not significantly different. Meanwhile, NSCLC patients treated with chemotherapy over non-chemotherapy were associated with longer survival (Table 2).
Table 2.
The 1-, 3-, 5-Year Survival Rate between 2013 and 2017 for LC after Diagnosis, by Cell Type and Chemotherapy Treatment at Srinagarind Hospital
| Variable | Number | Median time (95%CI) | 1-year Survival rate (95%CI) |
3-year Survival rate (95% CI) |
5-year Survival rate (95% CI) |
|---|---|---|---|---|---|
| Cell type | |||||
| SCLC | 43 | 0.58 (0.39 - 0.76) | 27.9 (15.57- 41.65) | 11.63 (4.26– 23.06) | NA |
| NSCLC | 1374 | 0.60 (0.53-0.66) | 37.8 (35.20- 40.34) | 15.14 (13.21- 17.19) | 11.9 (10.01 - 14.01) |
| SCLC with Chemotherapy | |||||
| Yes | 22 | 0.60 (0.32- 0.88) | 27.3 (11.12- 46.37) | NA | NA |
| No | 21 | 0.28 (0.03-0.53) | 28.6 (11.66- 48.18) | 14.3 (3.57-32.12) | NA |
| NSCLC with Chemotherapy | |||||
| Yes | 592 | 1.07 (0.97-1.17) | 53.5 (49.35- 57.40) | 17.9 (14.75- 21.28) | 11.8 (8.72- 15.34) |
| No | 782 | 0.33 (0.30-0.37) | 25.8 (22.79- 28.96) | 13.1 (10.78- 15.68) | 11.9 (9.52- 14.46) |
SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer; NA,Not applicable
Effect of targeted therapy on survival
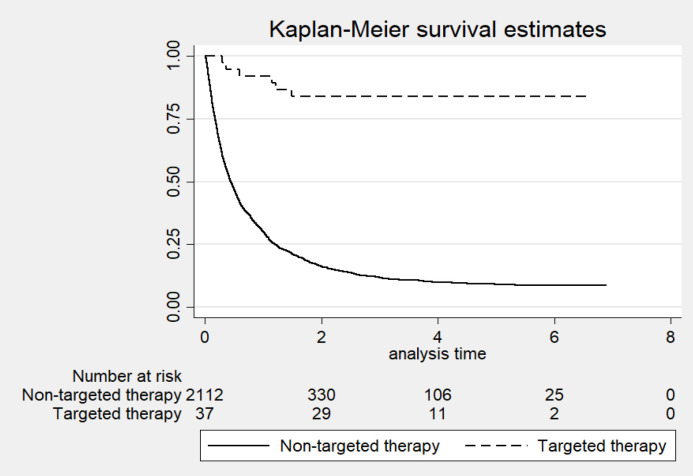
Treatment of patients with targeted over against non-targeted therapy was associated with longer survival (p-value < 0.001). The respective 1-, 3-, and 5-year OS rate for patients treated with targeted therapy vs. non-targeted therapy was 91.9% (95%CI: 76.93 to 97.31), 83.8% (95%CI: 67.42 to 92.37), and 83.8% (95%CI: 67.42 to 92.37). Meanwhile, the respective 1-, 3-, and 5-year OS rate for patients receiving non-targeted therapy was 30.1% (95%CI: 28.14 to 32.07), 11.8% (95%CI: 10.38 to 13.25), and 9.0% (95%CI: 7.69 to 10.52) (Figure 1).
Figure 1.
Overall Survival Based on Treatment of Patients (Targeted Therapy and Non-Targeted Therapy)
Survival and multivariable Cox regression analyses
After adjusting for sex, TNM stage, and histologic type, multivariable analysis of the entire cohort identified chemotherapy as an independent predictor of improved survival (adjusted HR= 0.48; 95% CI: 0.42 to 0.55; P < 0.001), and that sex, TNM stage, and histologic type were associated with survival (Table 3).
Table 3.
Multivariable Analysis between 2013 and 2017 for Overall Survival of the Entire LC Cohort after Diagnosis at Srinagarind Hospital
| Variable | Crude HR (95%CI) | Adjusted HR (95%CI) | p-value1 |
|---|---|---|---|
| 1. Sex | < 0.001 | ||
| Male | 1 | 1 | |
| Female | 0.74 (0.67 to 0.82) | 0.78 (0.68 to 0.89) | |
| 2. TNM stage | < 0.001 | ||
| Stage I and II | 1 | 1 | |
| Stage III | 3.76 (2.64 to 5.37) | 6.35 (4.05 to 9.95) | |
| Stage IV | 5.66 (4.01 to 7.98) | 8.32 (5.36 to 12.90) | |
| 3. Histologic type | < 0.001 | ||
| Squamous cell carcinoma | 1 | 1 | |
| Adenocarcinoma | 0.76 (0.63 to 0.91) | 0.78 (0.63 to 0.96) | |
| Small cell carcinoma | 0.98 (0.69 to 1.41) | 0.79 (0.50 to 1.22) | |
| Large cell carcinoma | 1.08 (0.88 to 1.32) | 1.01 (0.80 to 1.27) | |
| 4. Chemotherapy | < 0.001 | ||
| No | 1 | 1 | |
| Yes | 0.55 (0.49–0.60 ) | 0.48 (0.42–0.55) |
1p-value from partial likelihood ratio test, Adjusted HR, adjusting for Sex;TNM stage, Histologic type, and Chemotherapy
Discussion
The current study investigated the factors affecting the survival of LC patients after diagnosis at Srinagarind Hospital between 2013 and 2017. We describe these issues in detail for each topic.
Overall Survival of LC patients
The current study showed that the respective 1-, 3-, and 5-year survival rate was 31.2% (95% CI: 29.21 to 33.15%), 12.9 % (95%CI: 11.49 to 14.45), and 10.2% (95%CI: 8.74 to 11.70). As for the five-year net survival (%) estimates, the survival rate for LC from CONCORD was 10.2%. The rate is below 10.0% in Thailand, Brazil, Bulgaria, and India (Allemani et al., 2018). The population-based data from the SEER registries in the USA shows that the 5-year relative survival for LC was 18.4% between 2005 and 2011, compared to 12.2% between 1975 and 1977 when SEER record-keeping began (SEER, 2015 ).
Survival of LC by targeted therapy
Despite some improvement in survival among patients treated with targeted therapies, LC remains one of the most fatal cancers. Based on available data, the current study showed that the respective 1-, 3-, and 5-year survival rate between targeted therapy and non-targeted therapy was 91.9% (95% CI: 76.93 to 97.31 vs. 30.1% (95% CI: 28.14 to 32.07%), 83.8% (95% CI: 67.42 to 92.37) vs. 11.8% (95% CI: 10.37 to 13.25 %), and 83.8% (95% CI: 67.42 to 92.37) vs. 9.0 % (95% CI: 7.69 to 10.52%). The result is consistent with previous studies. Survival outcomes in patients with advanced non-small-cell lung cancer - treated with erlotinib - expanded access to the program data from Belgium (the TRUST study). Overall the respective survival rates at 1, 2, and 3 years was 26.4%, 10.9%, and 6.4% (Van Meerbeeck et al., 2014). Survival of patients with advanced NSCLC treated with first-generation EGFR-TKIs at a cancer hospital in Thailand (between 2011 and 2016). The respective overall observed survival rates of patients receiving EGFR-TKIs as first-line or maintenance (n=18), second-line (n=18), and third-line or more (n=14) was 15.9% (95%CI: 10.26 to 21.46), 10.9% (95%CI: 0.00 to 28.29), and 20.2% (95%CI: 6.26 to 34.21) (Sukauichai et al., 2018 ).
The respective 1- and 2-year observed survival rate of patients receiving Gefitinib was 85.0% and 57.9%. For patients receiving carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations, the respective 1- and 2-year survival NEJ002 (CBDCA/PTX) was 86.8% and 53.7% (Inoue et al., 2013).
Survival factors of LC patients
Sex
In Khon Kaen, Thailand, over the last 20 years, the trend incidence for LC has increased for both sexes. (Santong et al., 2018) Male/female is the one factor that may influence survival. Previous studies have reported that for most cancers being female provides a survival benefit over being male (Micheli et al., 2009; Cook et al., 2011), although females have a higher risk of death from bladder cancer (Zaitsu et al., 2015).
The current statistical work-up shows that male/female is a significant risk factor for LC patient survival. After adjusting all the variables in the model, the mortality risk of being female was 0.78–fold compared to male patients (adjusted HR=0.78, 95%CI: 0.68 to 0.89). The result is consistent with previous studies in the USA, confirming a female survival benefit over male for LC irrespective of histologic type. [30] Since then, other studies on male vs. female difference vis-à-vis LC survival have confirmed the trend (Sagerup et al., 2011; Li et al., 2019).
A similar survival benefit trend accrued to females after adjusting for years since cessation and smoking dose. The hazard ratio (HR) for LC mortality-comparing the association with smoking in women to that in men was 0.90 (adjusted HR=0.90, 95%CI: 0.80 to 0.90) for current smokers and 0.9 (adjusted HR=0.90, 95%CI: 0.90 to 1.00) for former smokers (Freedman et al., 2008).
TNM stage
The current study shows that the ‘stage of disease’ is a significant risk factor for LC patient survival. After adjusting for all variables in the model, ‘stage of disease’ confirmed a significant risk factor of patient survival. Stage IV was associated with an 8.32–fold mortality risk compared to stages I and II (adjusted HR=8.32; 95%CI: 5.36 to 12.90), and stage III had a 6.35–fold mortality risk compared to stages I and II (adjusted HR=6.35; 95%CI: 4.05 to 9.95).
The results agree with a previous study wherein, compared to Stage 1, the respective mortality risk of Stage IIIB/IV, Stage IIIA, and Stage II was 5.34–fold (adjusted HR=5.34; 95%CI: 4.95 to 5.76), 2.71–fold (adjusted HR=2.71; 95%CI: 2.49 to 2.95), and 1.80–fold (adjusted HR=1.80; 95%CI: 1.65 to 1.97) (Sculier et al., 2008). In contrast, pulmonary large cell carcinoma is an infrequent neoplasm with a poor prognosis for which ‘stage of disease’ had a weak association with increased mortality risk. In that study, compared to stage I, the respective increased mortality risk of Stage II, III, and IV was 1.28–fold (adjusted HR=1.28; 95% CI: 1.09 to 1.51), 1.82 (adjusted HR=1.82; 95% CI: 1.59 to 2.10), and 3.45 (adjusted HR=3.45; 95% CI: 3.01to 4.02) (Xiaochuan et al., 2020).
Histologic type
The current study showed that histology type was not a significant risk factor for LC survival. Compared to squamous cell carcinoma, the respective associated mortality risk for adenocarcinoma, small cell carcinoma, large cell carcinoma was 0.78–fold (adjusted HR=0.78; 95% CI: 0.63 to 0.96), 0.79–fold (adjusted HR=0.79; 95% CI: 0.50 to 1.22), and 1.01–fold (adjusted HR=1.01; 95% CI: 0.80 to 1.27). Compared to squamous cell carcinoma, the respective associated.
Consistent with a previous study, adenocarcinoma and large cell carcinoma were associated with a 0.97-fold (adjusted HR=0.97; 95%CI: 0.68 to 1.37) and a 1.01-fold (adjusted HR=0.01; 95%CI: 0.61 to 1.78] mortality risk compared to squamous cell carcinoma (Srisam-ang et al., 2005).
For mortality risk for NSCLC, adenocarcinoma and large cell was 0.96–fold (adjusted HR=0.96; 95% CI: 0.83 to 1.11), 0.95 (adjusted HR=0.95, 95%CI: 0.65 to 1.39) (Brzezniak et al., 2015).
Chemotherapy
The current study showed that chemotherapy afforded a significant advantaged vis-à-vis LC survival. After adjusting for all variables in the model, patients undergoing chemotherapy had improved survival. Chemotherapy was associated with a 0.48–fold mortality risk compared to non-chemotherapy (adjusted HR=0.48; 95%CI: 0.42 to 0.55). Our finding is consistent with prior studies. Chemotherapy alone was associated with a 0.55–fold mortality risk compared to no-treatment (adjusted HR=0.55; 95%CI: 0.54 to 0.56) (Lou et al., 2018). For advanced non-small cell lung cancer, chemotherapy alone was associated with a 0.38–fold mortality risk compared to no-treatment (adjusted HR=0.38; 95%CI: 0.37 to 0.39) and chemotherapy and surgery a 0.22–fold mortality risk compared to no-treatment (adjusted HR=0.22; 95%CI: 0.20 to 0.24) (David et al., 2016 ).
Advantages and Disadvantages of the study
To our knowledge, the current study is the most up-to-date examination of LC survival factors post-diagnosis between 2013 and 2017, according to the Srinagarind Hospital-Based Cancer Registry. Based on available data, targeted therapy significantly improved OS in LC patients of all ages, all cells types (NSCLC and SCLC), but further confirmatory research using extensive prospective clinical trials is needed. Novel targeted systemic therapies and the appropriate selection of LC patient treatments based on tumor molecular phenotypes and histologies should also be reviewed. The limitations of our study are the relatively small number of patients receiving targeted therapy.
In conclusion, the study confirmed that sex, stage of disease, histology, and chemotherapy are related to the survival of lung cancer patients. Primary prevention and screening for early detection are thus needed to improve survival. Factors affecting lung cancer survival in northeastern Thailand should focus on targeted therapy in any further investigations.
Author Contribution Statement
WM is a principal investigator and provided project management supervision. SK and CJ provided advice about the study design and statistical analyses. CS provided and supervised the interviewers, and assisted in assessing data quality. AP is a physician and operated on patients with apparent Lung cancer and assisted in the final diagnoses of the cases. SK was involved in exploratory analysis and data quality.
Acknowledgements
The authors thank (a) the KKU Cancer Unit staff for data collection and management, and (b) Mr. Bryan Roderick Hamman—under the aegis of the Publication Clinic, Khon Kaen University—for assistance with the English-language presentation of the manuscript.
Statement conflict of interest
The authors declare that they have no conflicts of interest.
References
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–75. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of progress. Chest. 2002;122:1037–57. doi: 10.1378/chest.122.3.1037. [DOI] [PubMed] [Google Scholar]
- Brzezniak C, Satram-Hoang S, Goertz HP, et al. Survival and racial differences of non-small cell lung cancer in the United States Military. J Gen Intern Med. 2015;30:1406–12. doi: 10.1007/s11606-015-3280-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev. 2011;20:1629–37. doi: 10.1158/1055-9965.EPI-11-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David EA, Canter RJ, Chen Y, Cooke DT, Cress RD. Surgical management of advanced non-small cell lung cancer is decreasing but is associated with improved survival. Ann Thorac Surg. 2016;102:1101–9. doi: 10.1016/j.athoracsur.2016.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson MK, Skosey C, Hoffman PC, et al. Sex-associated differences in presentation and survival in patients with lung cancer. J Clin Oncol. 1990;8:1402–7. doi: 10.1200/JCO.1990.8.8.1402. [DOI] [PubMed] [Google Scholar]
- Freedman ND, Leitzmann MF, Hollenbeck AR, Schatzkin A, Abnet CC. Cigarette smoking and subsequent risk of lung cancer in men and women: analysis of a prospective cohort study. Lancet Oncol. 2008;9:649–56. doi: 10.1016/S1470-2045(08)70154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002) Ann Oncol. 2013;24:54–9. doi: 10.1093/annonc/mds214. [DOI] [PubMed] [Google Scholar]
- Jazieh AR, Hussain M, Howington JA, et al. Prognostic factors in patients with surgically resected stages I and II non-small cell lung cancer. Ann Thorac Surg. 2000;70:1168–71. doi: 10.1016/s0003-4975(00)01529-0. [DOI] [PubMed] [Google Scholar]
- León-Atance P, Moreno-Mata N, González-Aragoneses F, et al. Multicenter analysis of survival and prognostic factors in pathologic stage I non-small-cell lung cancer according to the new 2009 TNM classification. Arch Bronconeumol. 2011;47:441–6. doi: 10.1016/j.arbres.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Li J, Zhu H, Sun L, Xu W, Wang X. Prognostic value of site-specific metastases in lung cancer: A population based study. J Cancer. 2019;10:3079–86. doi: 10.7150/jca.30463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Y, Dholaria B, Soyano A, et al. Survival trends among non-small-cell lung cancer patients over a decade: impact of initial therapy at academic centers. Cancer Med. 2018;7:4932–42. doi: 10.1002/cam4.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheli A, Ciampichini R, Oberaigner W, et al. 2009) The advantage of women in cancer survival: an analysis of EUROCARE-4 data. Eur J Cancer. 45:1017–27. doi: 10.1016/j.ejca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Sagerup CM, Småstuen M, Johannesen TB, Helland Å, Brustugun OT. Sex-specific trends in lung cancer incidence and survival: a population study of 40,118 cases. Thorax. 2011;66:301–7. doi: 10.1136/thx.2010.151621. [DOI] [PubMed] [Google Scholar]
- Santong C, Sriplung H, Phunmanee A, Pattanittum P. Trends and projections of lung cancer incidence in Khon Kaen province. Srinagarind Med J. 2018;33:222–8. [Google Scholar]
- Sculier JP, Chansky K, Crowley JJ, et al. The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the proposals for the 7th Edition. J Thorac Oncol. 2008;3:457–66. doi: 10.1097/JTO.0b013e31816de2b8. [DOI] [PubMed] [Google Scholar]
- Sriamporn S, Black RJ, Sankaranarayanan R, et al. Cancer survival in Khon Kaen Province, Thailand. Int J Cancer. 1995;61:296–300. doi: 10.1002/ijc.2910610303. [DOI] [PubMed] [Google Scholar]
- Sriplung H, Sontipong S, Martin N, et al. Cancer in Thailand Vol. III. 2003:1995–1997. [PubMed] [Google Scholar]
- Srisam-Ang K, Podhipak A, Narksawat K, Supaattagorn P, Tipayamongkholgul M. Survival of patients with advanced non-small-cell lung cancer at Ubon Ratchathani Cancer Center, Thailand. Southeast Asian J Trop Med Public Health. 2005;36:994–1006. [PubMed] [Google Scholar]
- Suwanrungruang K, Vatanasapt P, Kamsa-Ard S, Sriamporn S, Wiangnon S. Cancer survival in Khon Kaen, Thailand. IARC Sci Publ. 2011;162:211–6. [PubMed] [Google Scholar]
- Sukauichai S, Tovanabutra C, Wanglikitkul S, Chomprasert k. Survival of patients with advanced NSCLC treated with first-generation EGFRTKIs at a cancer hospital in Thailand, 2011-2016. J Med Oncl Ther. 2018;3:23–31. [Google Scholar]
- Van Meerbeeck J, Galdermans D, Bustin F, et al. Survival outcomes in patients with advanced non-small cell lung cancer treated with erlotinib: expanded access programme data from Belgium (the TRUST study) Eur J Cancer Care. 2014;23:370–9. doi: 10.1111/ecc.12146. [DOI] [PubMed] [Google Scholar]
- World Health Organization. International classification of diseases for oncology (ICD-O)– 3rd edition, 1st revision. 2013. [Accessed 12 March 2018]. Available: https://apps.who.int/iris/handle/10665/96612.
- Xiaochuan L, Jiangyong Y, Ping Z, Xiaonan W, Lin L. Clinical characteristics and prognosis of pulmonary large cell carcinoma: A population-based retrospective study using SEER data. Thorac Cancer. 2020;11:1522–32. doi: 10.1111/1759-7714.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsu M, Toyokawa S, Tonooka A, et al. Sex differences in bladder cancer pathology and survival: analysis of a population-based cancer registry. Cancer Med. 2015;4:363–70. doi: 10.1002/cam4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]