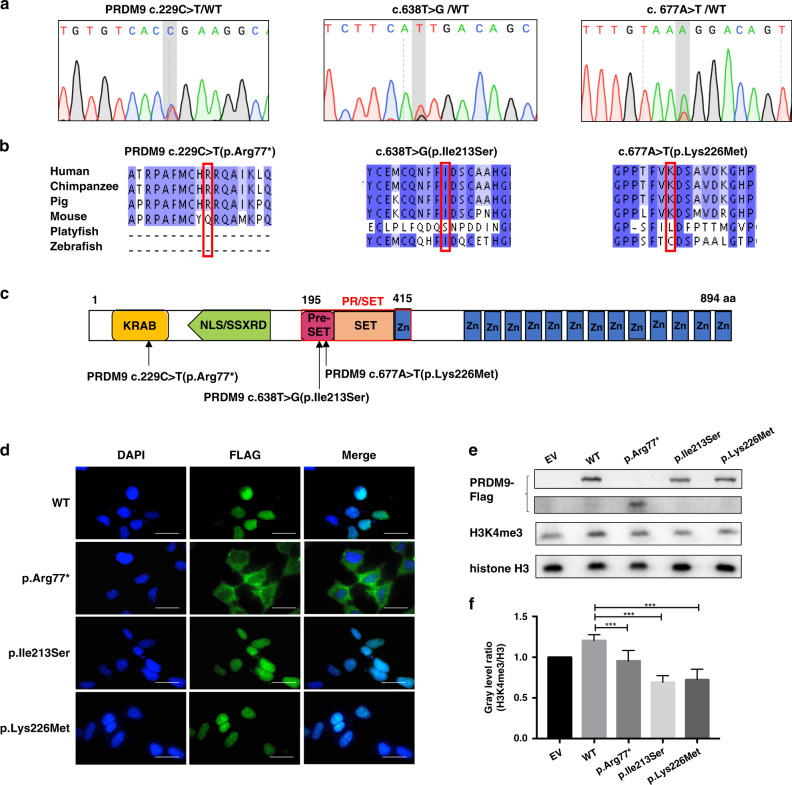
Fig. 1. Three pathogenic variants of PRDM9 identified in premature ovarian insufficiency (POI) patients affected its methyltransferase activity.
(a) Chromatograms of the three heterozygous variants. (b) The mutant amino acids were highly conserved in mammals. (c) PRDM9 c.229C>T (p.Arg77*) localized in the KRAB domain, before the nuclear localization signal (NLS) sequence; both PRDM9 c.638T>G (p.Ile213Ser) and PRDM9 c.677A>T (p.Lys226Met) localized on PR/SET domain (residues 195–415), which determined the methyltransferase activity of PRDM9. (d) HEK293 cells were transiently transfected with wild-type (WT) or mutant PRDM9 expression vectors, the subcellular location of PRDM9 protein were indicated by FLAG (green). Scale bar: 20 μm. (e) H3K4me3 was detected by western blot in HEK293 cells overexpressing empty vector (EV), wild-type (WT) or mutant PRDM9-FLAG. (f) The relative grayscale of H3K4me3 was calculated with the use of ImageJ, and compared between subgroups.