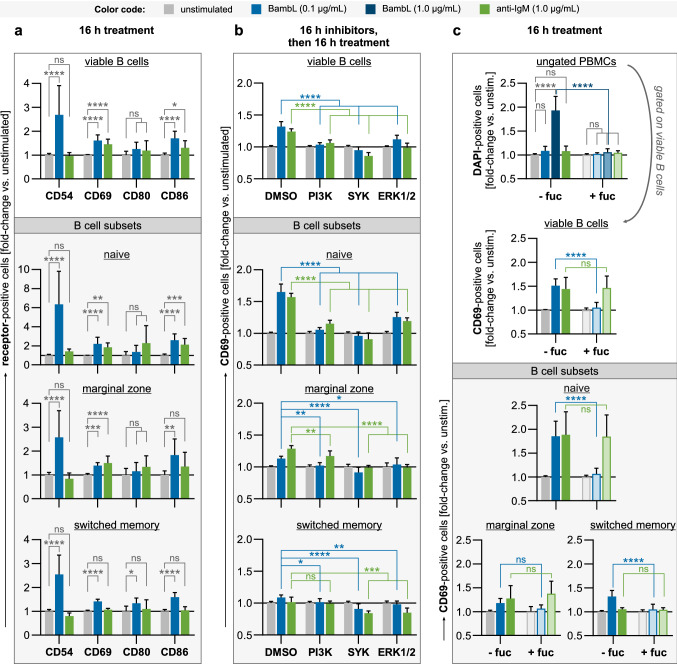
Fig. 3.
BambL stimulates the expression of classical activation markers, which is sensitive to inhibition of SYK, PI3K and ERK1/2. PBMCs were treated overnight and analyzed by flow cytometry. Viable B cells (DAPI− CD19+) and B cell subsets were identified within the samples after analysis as before. Bars are mean fold changes of signal-positive cell frequencies relative to unstimulated controls (error bars are SD). Statistical significance (ANOVA with Tukey’s correction): ns (p > 0.05), * (p ≤ 0.05), ** (p ≤ 0.01), *** (p ≤ 0.001), **** (p ≤ 0.0001). a BambL stimulates the expression of B cell activation markers. PBMCs (n = 9–10) were cultivated for 16 h in the presence of BambL, anti-IgM or left unstimulated. BambL elicited an upregulation of CD54, CD69 and CD86, which was most prominent in the naive B cell subset. b Inhibition of intracellular pathways diminishes B cell activation by BambL. B cells (n = 6–9) were first enriched from PBMCs and pre-treated with inhibitors against PI3K, SYK or ERK1/2 for 16 h (‘DMSO’: solvent controls), then stimulated and analyzed for surface expression of CD69 as before. c Excess fucose prevents cell death and activation. PBMCs (n = 9) were stimulated as in a, but 1 µg/mL BambL was included as an additional, cytotoxic stimulus in this experiment. A second set of treatment solutions was supplemented with L-fucose prior to the experiment (‘ + fuc’). The first panel shows the relative frequencies of all DAPI-positive cells (ungated PBMCs). Herein, viable B cells were identified and analyzed for their surface CD69 expression as before (second panel and subset panels). Samples exposed to 1 µg/mL lectin were excluded from the CD69 analyses because of their magnitude of cell death