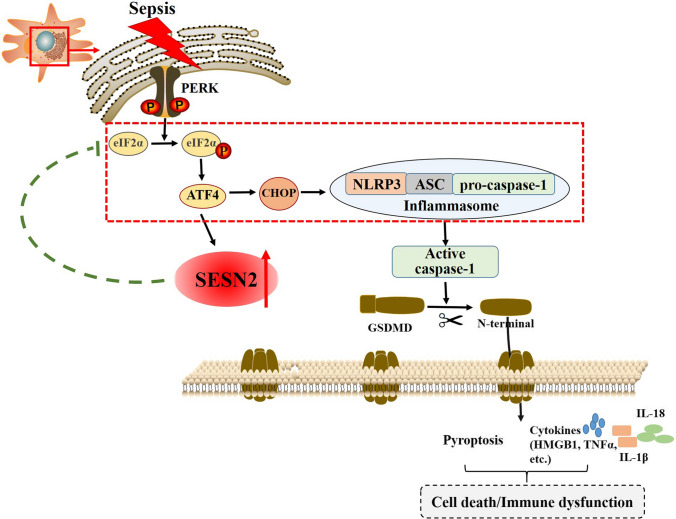
Fig. 9.
Basic schematic of the mechanism by which SESN2 inhibits pyroptosis and prolongs NLRP3 inflammasome activation by preserving ERS homeostasis. In splenic DCs, SESN2 expression was enhanced after CLP or stimulation with LPS via the PERK–ATF4–CHOP pathway. Sepsis per se induced severe latent ERS, and hyperactivation of ATF4 resulted in obvious elevation of CHOP expression, which might act as a potentiating step. Furthermore, CHOP activated the NLRP3 inflammasome and promoted the activation of pro-CASP-1 to CASP-1. Upon activation, CASP-1 cleaved the N-terminal fragment of GSDMD, and then GSDMD-N oligomerized in the plasma membrane and formed pores, resulting in the release of cellular contents and pyroptosis. Moreover, CASP-1 activation led to the maturation of IL-1β and IL-18 and the release of cytokines (IL-6, HMGB1, TNF-α, etc.), thereby contributing to the inflammatory response, immune dysfunction, and even cell death. Upregulation of SESN2 might reduce protein translation, and attenuate ERS, and subsequently suppress NLRP3 inflammasome activation through the PERK–ATF4–CHOP pathway, controlling the excessive inflammatory response and pyroptosis secondary to septic challenge