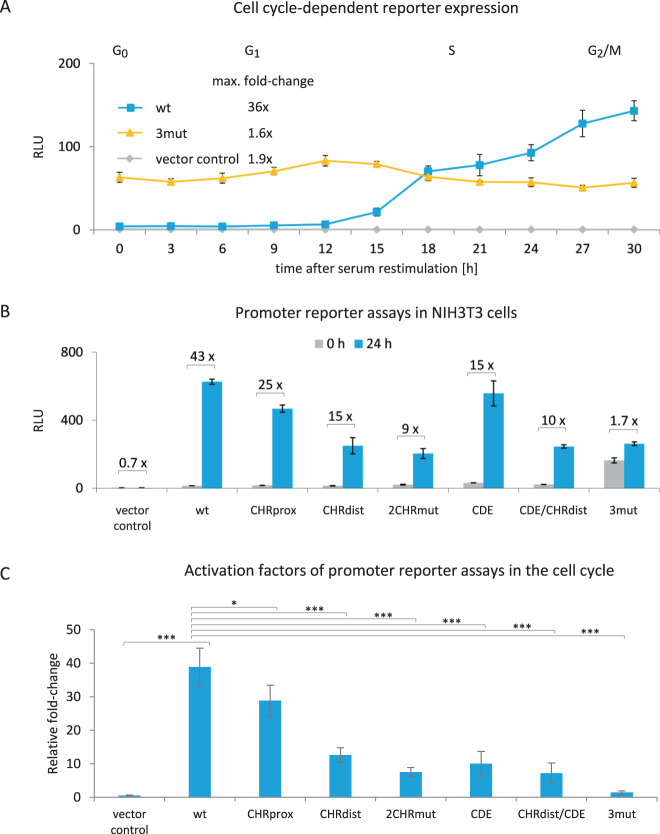
Fig. 3. Two CHR sites and one CDE control MKI67 cell cycle-dependent transcriptional regulation.
Luciferase reporter promoter assays from transfected cells that were serum-starved and restimulated by serum addition to the medium. Transfected plasmids were the empty reporter vector pGL4.10 (vector control) and wild-type or mutant reporter plasmid constructs based on a 596 bp MKI67 fragment including the TSS (see Fig. 2 and Suppl. Fig. 2 for position): wild-type (wt), mutant of the proximal CHR (CHRprox), mutant of the distal CHR (CHRdist), mutant of both CHR elements (2CHRmut), CDE mutant (CDE), a combination of mutations in CHRdist and CDE (CDE/CHRdist), and mutation of the three sites CHRprox, CHRdist, and CDE (3mut). Relative light units (RLUs) were calculated as the ratio of firefly luciferase activity from the promoter reporter constructs to the Renilla luciferase activity from a cotransfected control plasmid lacking a promoter. A Promoter reporter activity in different phases of the cell cycle. NIH3T3 cells were arrested in G0 through serum starvation for 72 h. To stimulate the cells to re-enter the cell cycle, serum was added to the medium. Luciferase assays from NIH3T3 cells transfected with vector control (gray), wt (blue), or 3mut (yellow) MKI67 firefly reporter promoter constructs. Cells were collected every 3 h after serum restimulation and RLUs were measured from three technical replicates for each time point. One representative experiment from two biological replicates is shown. The maximum fold-change for each construct was calculated. Approximate cell cycle phases were assessed by flow cytometry and are given above the graph. B NIH3T3 cells were serum-starved for 72 h (0 h, gray) and restimulated by serum addition (blue) for 24 h. One representative experiment from three biological replicates is shown. Cells were collected and RLUs were measured as technical duplicates. The relative fold-change in RLUs from 0 to 24 h for each promoter construct was calculated and is given above the columns. For all graphs, mean values ± SD are given. C Compilation of relative activation factors for each tested plasmid from experiments as shown in B. Averages of activation factors of RLUs 24 h after restimulation over RLUs at 0 h before stimulation. Activation factors yield a rate of activation for each promoter construct in the cell cycle. Results are from n ≥ 3 biological replicates for each plasmid examined. Significance was tested via an unpaired t-test (n.s., not significant; *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001).