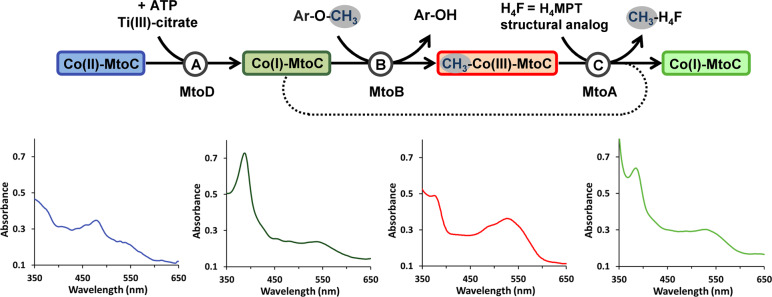
Fig. 4. O-demethylation and methyl transfer conducted by Mto proteins.
Reaction A: MtoD, ATP and titanium (III) citrate are required for activation of MtoC from the Co(II) state (blue) to the active Co(I) state (dark green). Reaction B: MtoB transfers the methyl group of the methoxy compound (Ar-O-CH3) to Co(I)-MtoC resulting in methylated Co(III)-MtoC (red). The MtoB activities with 2-methoxybenzoate (MB) and TMB are shown in Fig. S2A. With methanol or trimethylamine as substrate no activity could be observed. Conversion of TMB to 3-OH-4,5-dimethoxybenzoate was confirmed by HPLC (Fig. S3). Reaction C: For measuring MtoA activity, the H4MPT structural analog H4F was used. We got strong evidence that MtoA transfers the methyl group from methylated Co(III)-MtoC (red) to H4F thereby producing Co(I)-MtoC (light green). The activity is shown in Fig. S2B. In M. shengliensis H4MPT and not H4F is most likely the methyl group acceptor as M. shengliensis does not have the genomic capacity to synthesize H4F. Also, the methyl-transfer reaction is not occurring if CoM is used instead of H4F (Fig. S2B). All bottom panels correspond to UV/visible spectra measured after each reaction reflecting the different states of the cobalamin carried by MtoC.