Abstract
Introduction
Diabetes is a chronic and progressive disease that usually causes disrupts the function of the body's organs and can eventually lead to cardiomyopathy, nephropathy, retinopathy, and neuropathy. Diabetic nephropathy (DN) is the most common cause of chronic kidney disease and causes chronic structural changes in different parts of the affected kidney. Glycocalyx layer is one of the most important components of the vascular base found in the endothelium throughout the body's arteries and it has been shown that glycocalyx is also damaged during diabetic nephropathy. Our goal is to conduct this systematic review study is to find the cause-and-effect relationship between glycocalyx and diabetic nephropathy and also to clarify the role of the endothelial renal glycocalyx in understanding of mechanism of the course of diabetic nephropathy, and to provide an accurate background for further important studies.
Methods
All databases included MEDLINE (PubMed), Science Direct, Scopus, Ovid and Google Scholar were systematically searched for related published articles. In all databases, the following search strategy was implemented and these key words (in the title/abstract) were used: “diabetes” AND “glycocalyx” OR “diabetic nephropathy” AND “glycocalyx”.
Results and discussion
A total of 19 articles were retrieved from all databases using search strategy. After screening based on the title and abstract, number of 17 of them selected for full text assessment. Finally, after extracting the key points and making connections between the articles, we came up with new points to consider. It can be said that diabetes with the action of reactive oxygen species through oxidative stress, increases ICAM-1 and TNF-α and decreases heparanase enzyme, it affects the glomerular endothelium and eventually leads to albuminuria and destruction of the Glx layer.
Conclusion
Diabetes causes super-structural changes in the kidney nephrons at the glomerular level. The glomerular filter barrier, which includes the epithelial cell called the podocyte, endothelial pore cells, and basal membrane of the glomerulus, plays a major role in stabilizing the selective glomerular function in healthy individuals. Diabetic nephropathy also causes changes in endothelial glycocalyx.
Keywords: Glycocalyx, Diabetes, Nephropathy, A commentary
Introduction
Diabetes is a chronic and progressive disease that, by damaging the vascular endothelium of various organs in the body, increases the risk of premature death, organ failure, and disability [1]. Chronic diabetes usually causes micro and macroangiopathy, which in turn disrupts the function of the body's organs, especially the heart, kidneys, retina, peripheral and central nervous systems, and can eventually lead to cardiomyopathy, nephropathy, retinopathy, and neuropathy [2–5].
Diabetic nephropathy (DN) is the most common cause of chronic kidney disease (CKD). This complication has a progressive course and gradually leads to end-stage renal disease (ESRD) [6]. Patients with ESRD may eventually need a kidney transplant or dialysis, and in addition to physical disabilities, they will be subject to heavy economic pressures [7]. As a result, recent research in diabetic nephropathy has focused on new diagnostic and therapeutic goals to prevent the progression of the disease by early diagnosis and effective treatment [8].
Diabetic nephropathy causes chronic structural changes in different parts of the affected kidney. Although tubular-interstitial damage is an important component of diabetic kidney damage, the first changes in diabetes occur in treated glomeruli in the form of increased extracellular fluid and fibrosis and thickening of the capillary membrane [9]. Several studies have shown that oxidative stress plays an important role in the occurrence of such problems and nephrotoxicity [10].
One of the most important components of the vascular base found in the endothelium throughout the body's arteries is the vascular glycocalyx layer. This layer is a network of proteoglycans and glycosaminoglycans involved in various physiological processes. Heparin sulfate (the most glycosaminoglycan), chondroitin sulfate, dermatan sulfate, and hyaluronan are among the most important vascular glycocalyx layer proteoglycans. Glycosaminoglycans have a negative charge on the glycocalyx (Glx) layer, so they can act as an electrical barrier against material passing through the capillary wall. Vascular Glx is a binding site for enzymes and other important endothelial elements and is involved in the transmission of mechanical force, the guidance of intercellular messages, and in inflammatory processes [11].
Traditionally, microalbuminuria has been used as the first biomarker of diabetic nephropathy. However, due to the progressive nature of this complication, researchers are looking for indicators that can detect kidney involvement before the onset of microalbuminuria. One of these new markers is glycosaminoglycans, which are essential components of the Glx structure [12].
Given the clinical significance and high prevalence of diabetic nephropathy and the difficulties in coping with it, it is essential to find new diagnostic and therapeutic approaches to early detection and correction of injuries caused by diabetic nephropathy. Recently, the prominent role of endothelial glycocalyx (eGlx) has been shown as a valuable study clue in various vascular pathologies. Due to the diabetic pandemic and the increasing prevalence of nephropathy, it is necessary to identify the factors affecting its development and effective treatments. Our goal is to conduct this systematic review study is to find the cause-and-effect relationship between Glx and diabetic nephropathy and also to clarify the role of the endothelial renal Glx in understanding of mechanism of the course of diabetic nephropathy, and to provide an accurate background for further important studies.
The role and relationship of oxidative stress as a factor in activating signaling pathways of processes such as apoptosis, inflammation and cell response to stress [13], with the development of diabetic nephropathy and Glx destruction will also be investigated.
Methods
All databases included MEDLINE (PubMed), Science Direct, Scopus, Ovid and Google Scholar were systematically searched for related published articles. In all databases, the following search strategy was implemented and these key words (in the title/abstract) were used: “diabetes” AND “glycocalyx” OR “diabetic nephropathy” AND “glycocalyx”. Our search included in vitro conditions, computer models, animal models, and human models. The audit of these articles was based on the validity, journal IF and the specialized relationship of the article with our topic. After fully justifying the members of the group and dividing the responsibility, each member, in accordance with his / her assigned responsibility, carefully identified all the articles searched with the mentioned keywords and notified the relevant results in the next meetings. After discussing the relevant contents between the members, the related contents were separated by specific headings and replaced by headings in the original article. After writing the original text, the article was read many times by all the authors and finally, after editing, it was ready to be presented to the journal.
Results and discussion
A total of 19 articles were retrieved from all databases using search strategy. After screening based on the title and abstract, number of 17 of them selected for full text assessment. Finally, after extracting the key points and making connections between the articles, we came up with new points to consider.
eGlx and its structure
Endothelial glycocalyx (eGlx) is the first protein barrier in blood vessels. Damage to eGlx is seen in both type 1 and type 2 diabetes [14]. A wide range of membrane-bound macromolecules, including glycoproteins containing acidic oligosaccharides and terminal sialic acid (SA), as well as proteoglycans together with the associated glycosaminoglycan (GAG) side chains, form the endothelial cells (EC) [15]. Components that attach to this biopoly matrix include plasma proteins, enzymes, enzyme inhibitors, growth factors, and cytokines, through cationic sites in their structure, as well as cationic amino acids, cations, and water [16, 17].
GAGs are heteropolysaccharides characterized by single disaccharide repeats [18]. Hyaluronic acid (HA), heparan, chondroitin, creatine and dermatan sulfates are GAG types [19, 20]. Among these, heparan sulfate has the highest amount, which is 50–90%. Small changes in the concentration and organization of GAGs can greatly affect eGCX function because GAGs contain different binding sites for plasma-derived proteins [21, 22].
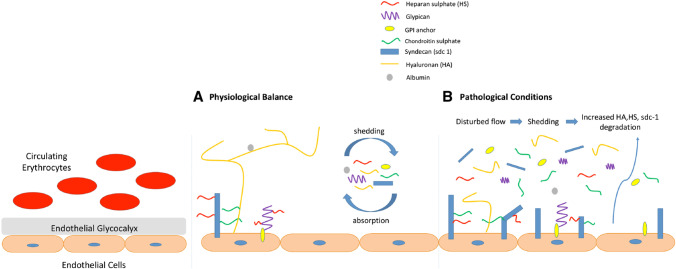
Syndecans-1, expressed in ECs, has three GAG junctions that are essentially replaced by HS [23]. The only glypican expressed in ECs is glypican-1. Close to the membrane, three to four of its GAG junctions are replaced exclusively by HS. Glypican-1 binds directly to plasma plas membranes via a glycosylphosphatidylinositol (GPI) anchor [23, 24]. The image that integrates all of the eGlx components described above with a range of functions including endothelial cell adhesion, uptake and cell signaling is shown in Fig. 1 (adapted from Reference [14, 22]).
Fig. 1.
Structure and functions of eGlx in pathophysiology
eGlx disorders in diabetic nephropathy and its pathophysiology role
Chronic hyperglycemia damages the vessel wall and increases endothelial permeability by impaired NO synthase function [20]. Leakage of protein, such as albumin, into the glomerular capillaries leads to albumin in the urine (albuminuria), a symptom of kidney disease. Sara Desideri et al. showed that glomerular eGlx damage is directly related to increased glomerular albumin permeability. Experimental models show glomerular endothelial cell loss. Vascular endothelial growth factor (VEGF) A is also regulated in primary diabetes, which is associated with albuminuria [14]. According to a cross-sectional study in which Glx thickness was assessed by 5 measurements with GlycoCheck, the data showed that higher perfused boundary region (PBR) indicates smaller Glx width. Albuminuria and PBR are directly related. In diabetic patients with a long history of albuminuria, PBR is significantly higher and shows impaired Glx [25].
Boels et al. also showed that continuous repair of glomerular eGlx in diabetic nephropathy is possible by inhibition of Monocyte Chemotactic Protein-1 (MCP-1). On the other hand, heparan sulfate is a major component of Glx. The expression of heparanase enzyme is of particular importance for the analysis of the mechanism of degradation and repair of Glx. Diabetic mice also showed an increase in glomerular heparanase protein expression compared to non-diabetic Apoe KO mice, indicating that in a diabetic environment, Glx is degraded in a heparanase-dependent manner [26].
Endothelin receptor antagonists, such as atrasentan, can reduce albuminuria in patients with diabetic nephropathy and restore the glomerular eGlx lining. By examining the mechanisms of atrasentan, Allison et al. Showed that the effect of atrasentan depends on the regulation of heparanase enzyme levels. Treatment with atrastan for 4 weeks in diabetic rats reduced progressive albuminuria without significant change in glomerular capillary size or podocyte count. In fact, diabetic nephropathy is associated with glomerular inflammation, where macrophages and endothelial cells appear to increase heparanase levels [27].
It is important to note that podocyte cells, which are epithelial cells that play a key role in the health of basement membrane function, increase in the urine during glomerular irritation. Evaluation of podocytes and related proteins in urine is directly related to the severity of diabetic nephropathy [28]. Progression of diabetic kidney disease is initially evident as dilatation of the mesangial matrix following podocytes loss, glomerular volume expansion, and severe albuminuria [29].
Another study showed that diabetes causes oxidative stress by measurement of Malondialdehyde (MDA) and fluorescence recovery after photobleaching (FRAP) levels. The expression levels of Intercellular Adhesion Molecule 1 (ICAM-1) and Tumor Necrosis Factor alpha (TNF-α) also increased in diabetic rats with kidney damage. In the kidney, TNF-α reduces blood flow and glomerular filtration rate and increases glomerular permeability to albumin [30].
In view of all the above, it can be said that eGlx is a complex network of proteoglycans, glycoproteins and other soluble compounds that include vascular endothelium and in many physiological processes including vascular permeability, shear stress transduction and the interaction of blood cells and other molecules with the vessel wall plays an important role. Also with the knowledge that diabetes through various molecular pathways and especially through oxidative stress, disrupts some physiological mechanisms [21], it can be said that diabetes with the action of reactive oxygen species through oxidative stress, increases ICAM-1 and TNF-α and decreases heparanase enzyme, it affects the glomerular endothelium and eventually leads to albuminuria and destruction of the Glx layer. A summary of the cause-and-effect relationship between eGlx and diabetic nephropathy is shown in Table 1.
Table 1.
Relationship between diabetes and destruction of glycocalyx
| Sr. No | Author/year | Journal | Database | Study type | Conclusion |
|---|---|---|---|---|---|
| 1 | Wang/ 2020 | Biochimica et Biophysica Acta (BBA)—Molecular Basis of Disease | Science Direct | Experimental | Loss of podocytes → glomerular volume expansion → severe albuminuria |
| 2 | Desideri/ 2019 | Biorheology | Pubmed | Review | VEGF → albuminuria / albuminuria → eGlx damage |
| 3 | Korakas/ 2019 | Current Vascular Pharmacology | Pubmed | Review | Diabetes → oxidative stress → albuminuria → destruction of the glycocalyx layer |
| 4 | Petrica/ 2017 | Diabetology & Metabolic Syndrome | Pubmed | Cross-Sectional | Podocytes levels → proteins in urine → severity of diabetic nephropathy |
| 5 | Boels/ 2017 | The American Journal of Pathology | Science Direct/ Pubmed | Experimental | expression of heparanase enzyme → degradation and repair of glycocalyx |
| 6 | Najafi/ 2017 | Nephrology | Pubmed | Experimental | Diabetes → oxidative stress → increasing ICAM-1 & TNF-α / TNF-α → increasing glomerular permeability to albumin |
| 7 | Winther/ 2017 | Artery Research | Science Direct | Cross-Sectional | Higher PBR → Smaller glycocalyx width / Higher albuminuria → Higher PBR → impaired glycocalyx |
| 8 | Allison/ 2016 | American Diabetes Association | Pubmed | Experimental | regulation of heparanase enzyme → reduced progressive albuminuria |
An overview of the relationship between the points extracted and other articles: Diabetes causes super-structural changes in the kidney nephrons at the glomerular level through oxidative stress and causes changes in eGlx
Conclusion
So far, no study has examined the cause-and-effect relationship between the Glx layer and diabetes, but several different articles have examined the background role. Diabetes causes super-structural changes in the kidney nephrons at the glomerular level. The glomerular filter barrier, which includes the epithelial cell called the podocyte, endothelial pore cells, and basal membrane of the glomerulus, plays a major role in stabilizing the selective glomerular function in healthy individuals. Albuminuria, which is a direct result of a defect in the glomerular filter barrier and an early manifestation of diabetic nephropathy, may be associated with progression to ESRD. In a general conclusion, it can be stated that diabetic nephropathy, with the help of oxidative stress, albuminuria, changes in the level of podocytes and finally changes in structural enzymes, causes changes in the thickness of eGlx and its disappearance.
Acknowledgements
Authors would like to thank Arak University of Medical Sciences for supporting this study (Funding No. 2807).
Abbreviations
- DM
Diabetes mellitus
- DN
Diabetic nephropathy
- ESRD
End-stage renal disease
- CKD
Chronic kidney disease
- eGlx
Endothelial glycocalyx
- EC
Endothelial cells
- GAG
Glycosaminoglycan
- VEGF
Vascular endothelial growth factor
- PBR
Perfused boundary region
- MCP-1
Monocyte Chemotactic Protein-1
- MDA
Malondialdehyde
- FRAP
Fluorescence recovery after photobleaching
- ICAM-1
Intercellular Adhesion Molecule 1
- TNF-α
Tumor Necrosis Factor alpha
Declarations
Ethical approval
The ethical approval of this study was granted by the Medical Ethics Committee, Arak University of Medical Sciences No. IR.ARAKMU.REC.1396.131.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang WC, Cao HL, Wang YZ, Li TT, Hu HY, Wan Q, et al. Inhibition of nitric oxide synthase aggravates brain injury in diabetic rats with traumatic brain injury. Neural Regen Res. 2021;16(8):1574–81. [DOI] [PMC free article] [PubMed]
- 2.Habib AA, Brannagan TH. Therapeutic strategies for diabetic neuropathy. Curr Neurol Neurosci Rep. 2010;10(2):92–100. doi: 10.1007/s11910-010-0093-7. [DOI] [PubMed] [Google Scholar]
- 3.Gupta A, Gupta P, Biyani M. Targeted therapies in diabetic nephropathy: an update. J Nephrol. 2011;24(6):686–695. doi: 10.5301/jn.5000041. [DOI] [PubMed] [Google Scholar]
- 4.Sytze Van Dam P, Cotter MA, Bravenboer B, Cameron NE. Pathogenesis of diabetic neuropathy: focus on neurovascular mechanisms. Eur J Pharmacol. 2013;719(1):180–6. doi: 10.1016/j.ejphar.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Dogné S, Flamion B, Caron N. Endothelial glycocalyx as a shield against diabetic vascular complications: involvement of hyaluronan and hyaluronidases. Arterioscler Thromb Vasc Biol. 2018;38(7):1427–1439. doi: 10.1161/ATVBAHA.118.310839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donate-Correa J, Luis-Rodríguez D, Martín-Núñez E, Tagua VG, Hernández-Carballo C, Ferri C, et al. Inflammatory targets in diabetic nephropathy. J Clin Med. 2020;9(2):458. doi: 10.3390/jcm9020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Brenneman J, Hill J, Pullen S. Emerging therapeutics for the treatment of diabetic nephropathy. Bioorg Med Chem Lett. 2016;26(18):4394–4402. doi: 10.1016/j.bmcl.2016.07.079. [DOI] [PubMed] [Google Scholar]
- 9.Shao D, Liu J, Ni J, Wang Z, Shen Y, Zhou L, et al. Suppression of XBP1S mediates high glucose-induced oxidative stress and extracellular matrix synthesis in renal mesangial cell and kidney of diabetic rats. PLoS One. 2013;8(2):e56124. doi: 10.1371/journal.pone.0056124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Changizi-Ashtiyani S, Seddigh A, Najafi H, Hossaini N, Avan A, Akbary A, et al. Pimpinella anisum L. ethanolic extract ameliorates the gentamicin-induced nephrotoxicity in rats. Nephrology. 2017;22(2):133–8. doi: 10.1111/nep.12953. [DOI] [PubMed] [Google Scholar]
- 11.Ushiyama A, Kataoka H, Iijima T. Glycocalyx and its involvement in clinical pathophysiologies. J Intensive Care. 2016;4(1):1–11. doi: 10.1186/s40560-016-0182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S-Y, Choi ME. Urinary biomarkers for early diabetic nephropathy: beyond albuminuria. Pediatr Nephrol. 2015;30(7):1063–1075. doi: 10.1007/s00467-014-2888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Changizi-Ashtiyani S, Najafi H, Firouzifar MR, Shafaat O. Grape seed extract for reduction of renal disturbances following reperfusion in rats. Iran J Kidney Dis. 2013;7(1):28. [PubMed] [Google Scholar]
- 14.Desideri S, Onions KL, Baker SL, Gamez M, El Hegni EHH, Russell A, et al. Endothelial glycocalyx restoration by growth factors in diabetic nephropathy. Biorheology. 2019;56(2–3):163–179. doi: 10.3233/BIR-180199. [DOI] [PubMed] [Google Scholar]
- 15.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440(5):653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 16.Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68(1):729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 17.Osterloh K, Ewert U, Pries AR. Interaction of albumin with the endothelial cell surface. Am J Physiol Heart Circ Physiol. 2002;283(1):H398–H405. doi: 10.1152/ajpheart.00558.2001. [DOI] [PubMed] [Google Scholar]
- 18.Jackson RL, Busch SJ, Cardin AD. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev. 1991;71(2):481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- 19.Oohira A, Wight TN, Bornstein P. Sulfated proteoglycans synthesized by vascular endothelial cells in culture. J Biol Chem. 1983;258(3):2014–2021. doi: 10.1016/S0021-9258(18)33090-4. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz O, Afsar B, Ortiz A, Kanbay M. The role of endothelial glycocalyx in health and disease. Clin Kidney J. 2019;12(5):611–619. doi: 10.1093/ckj/sfz042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korakas E, Ikonomidis I, Markakis K, Raptis A, Dimitriadis G, Lambadiari V. The endothelial glycocalyx as a key mediator of albumin handling and the development of diabetic nephropathy. Curr Vasc Pharmacol. 2020;18(6):619–31. [DOI] [PubMed]
- 22.Yilmaz O, Afsar B, Ortiz A, Kanbay M. The role of endothelial glycocalyx in health and disease. Clin Kidney J. 2019;12(5):611– 19. [DOI] [PMC free article] [PubMed]
- 23.Rosenberg RD, Shworak NW, Liu J, Schwartz JJ, Zhang L. Heparan sulfate proteoglycans of the cardiovascular system. Specific structures emerge but how is synthesis regulated? J Clin Investig. 1997;99(9):2062–70. doi: 10.1172/JCI119377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fransson L-Å, Belting M, Cheng F, Jönsson M, Mani K, Sandgren S. Novel aspects of glypican glycobiology. Cell Mol Life Sci. 2004;61(9):1016–1024. doi: 10.1007/s00018-004-3445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winther SA. Reduced sublingual endothelial glycocalyx in type 1 diabetics with diabetic nephropathy. Artery Research. 2017;20:59. doi: 10.1016/j.artres.2017.10.048. [DOI] [Google Scholar]
- 26.Boels MGS, Koudijs A, Avramut MC, Sol WMPJ, Wang G, van Oeveren-Rietdijk AM, et al. systemic monocyte chemotactic protein-1 inhibition modifies renal macrophages and restores glomerular endothelial glycocalyx and barrier function in diabetic nephropathy. Am J Pathol. 2017;187(11):2430–2440. doi: 10.1016/j.ajpath.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Allison SJ. Diabetic nephropathy: atrasentan stabilizes the endothelial glycocalyx. Nat Rev Nephrol. 2016;12(6):315. doi: 10.1038/nrneph.2016.53. [DOI] [PubMed] [Google Scholar]
- 28.Petrica L, Ursoniu S, Gadalean F, Vlad A, Gluhovschi G, Dumitrascu V, et al. Urinary podocyte-associated mRNA levels correlate with proximal tubule dysfunction in early diabetic nephropathy of type 2 diabetes mellitus. Diabetol Metab Syndr. 2017;9(1):31. doi: 10.1186/s13098-017-0228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W, Jiang S, Tang X, Cai L, Epstein PN, Cheng Y, et al. Sex differences in progression of diabetic nephropathy in OVE26 type 1 diabetic mice. Biochim Biophys Acta (BBA) Mol Basis Dis. 2020;1866(1):165589. doi: 10.1016/j.bbadis.2019.165589. [DOI] [PubMed] [Google Scholar]
- 30.Mahmoudzadeh L, Najafi H, Changizi-Ashtiyani S, Yarijani ZM. Anti-inflammatory and protective effects of saffron extract in ischaemia/reperfusion-induced acute kidney injury. Nephrology. 2017;22(10):748–754. doi: 10.1111/nep.12849. [DOI] [PubMed] [Google Scholar]