Abstract
Objectives. To quantify disparities in health and economic burdens of cancer attributable to suboptimal diet among US adults.
Methods. Using a probabilistic cohort state-transition model, we estimated the number of new cancer cases and cancer deaths, and economic costs of 15 diet-related cancers attributable to suboptimal intake of 7 dietary factors (a low intake of fruits, vegetables, dairy, and whole grains and a high intake of red and processed meats and sugar-sweetened beverages) among a closed cohort of US adults starting in 2017.
Results. Suboptimal diet was estimated to contribute to 3.04 (95% uncertainty interval [UI] = 2.88, 3.20) million new cancer cases, 1.74 (95% UI = 1.65, 1.84) million cancer deaths, and $254 (95% UI = $242, $267) billion economic costs among US adults aged 20 years or older over a lifetime. Diet-attributable cancer burdens were higher among younger adults, men, non-Hispanic Blacks, and individuals with lower education and income attainments than other population subgroups. The largest disparities were for cancers attributable to high consumption of sugar-sweetened beverages and low consumption of whole grains.
Conclusions. Suboptimal diet contributes to substantial disparities in health and economic burdens of cancer among young adults, men, racial/ethnic minorities, and socioeconomically disadvantaged groups. (Am J Public Health. 2021;111(11):2008–2018. https://doi.org/10.2105/AJPH.2021.306475)
Cancer is a major public health burden and the second leading cause of death in the United States, with approximately 1.8 million new cancer cases and 0.6 million cancer deaths estimated in 2018.1 The annual numbers of new cancer cases and deaths are expected to increase, reaching 2.3 million and 1.0 million, respectively, in 2040.2 The direct medical cost associated with cancer care was estimated to increase from $124 billion in 2010 to $173 billion in 2020, a 17% increase over 10 years.3 Reducing cancer burdens through effective prevention strategies has long been an overarching goal for public health policies in the United States.
Suboptimal diet is well known to be associated with the risk of cancer. Strong evidence from systematic reviews suggests that a high consumption of processed and red meats and a low consumption of whole grains and dairy products are associated with an increased risk of colorectal cancer, and a low consumption of fruits and vegetables is associated with an increased risk of cancer in the oral cavity, pharynx, and larynx.4,5 Importantly, obesity has been recognized as a risk factor for 13 types of cancers.6 Sugar-sweetened beverage (SSB) consumption can increase the risk of obesity-associated cancers by contributing to weight gain and obesity.7,8 We have previously estimated that more than 80 000 new cancer cases among US adults each year are attributable to suboptimal intake of these dietary factors.9 Diet-associated cancers are likely to contribute to a substantial economic burden given the high costs of cancer care. Optimizing dietary intake could be a cost-effective strategy for cancer prevention, yet the economic burden of diet-attributable cancers has not been quantified.
In addition, cancer disproportionally affects individuals of low socioeconomic status and non-Hispanic Blacks in the United States, who bear a higher rate of cancer incidence and death for many cancers than other population subgroups.10 Meanwhile, dietary disparities have persisted or worsened for most dietary components among US adults despite an overall modest improvement in Americans’ diet in the past 10 to 15 years.11–13 For example, when low-income adults who participated in the Supplemental Nutrition Assistance Program (SNAP) were compared with higher-income individuals, SNAP participants experienced no improvements in diet quality from 2003 to 2013, whereas diet quality significantly improved among higher-income individuals.12 Non-Hispanic Blacks had a worse diet quality and smaller improvement in diet quality over time compared with non-Hispanic Whites.11,13 Interestingly, the racial/ethnic disparities in colorectal cancer incidence and mortality parallel the racial/ethnic disparities in diet quality among US adults.10,14,15 Dietary disparities could contribute to substantial cancer disparities in the United States. In the present study, we aimed to quantify disparities in the health and economic burdens of cancer attributable to suboptimal diet among US adults. Such findings can inform priority areas in public health strategies to improve diet and reduce diet-attributable cancer disparities in the United States.
METHODS
We used a cohort state-transition model, the Dietary and Cancer Outcome Model,16 to estimate the health and economic burdens of cancer attributable to suboptimal diet among US adults over a lifetime in a closed cohort (Figure A and Method A, available as supplements to the online version of this article at http://www.ajph.org). Starting from a cancer-free representative population of US adults in 2017, the model simulated the development and progression of cancer over a lifetime by transitions of health states and tracked numbers of cancer cases and cancer deaths, and associated direct medical and indirect societal costs that occurred annually in the cohort. To estimate diet-associated cancer burdens, the model compared the incremental difference in cancer burdens between the current and optimal dietary intake scenarios. Diet-associated cancer burdens were estimated in population subgroups stratified by age, gender, race/ethnicity, education, income, and SNAP participation, and combined for estimates among US adults. Health outcomes and economic costs were discounted at 3% annually as recommended by the Second Panel on Cost-Effectiveness in Health and Medicine.17
Study Population
Demographics were obtained on noninstitutionalized US adults aged 20 years or older based on the 2 most recent cycles of the National Health and Nutrition Examination Survey (NHANES) (2015–2016 and 2017–2018; Table A, available as a supplement to the online version of this article at http://www.ajph.org). Population subgroups were jointly stratified by age (20–44, 45–54, 55–64, and 65 years or older), gender (men and women), race/ethnicity (non-Hispanic Whites, non-Hispanic Blacks, Hispanics, and others), education (< high school, high school, some college, and college graduate or above), income (family income to poverty ratio [FIPR], calculated by using the poverty guideline by the Department of Health and Human Services, of < 1.3, 1.3–2.9, and ≥ 3), and SNAP participation status (SNAP participants, SNAP-eligible nonparticipants, and SNAP ineligible individuals). Information on race/ethnicity was self-reported according to fixed categories; Asian and other racial/ethnic groups were combined into 1 group because of their small sample sizes.18
Current and Optimal Dietary Intakes
Seven dietary factors representing the suboptimal diet (a low consumption of whole grains, dairy products, fruits, and vegetables, and a high consumption of red meats, processed meats, and SSBs) were selected on the basis of evidence from systematic reviews performed by the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) and others showing “convincing” or “probable” evidence of association with cancer risk.8 We estimated current intakes of these dietary factors by using two 24-hour dietary recalls per person from NHANES cycle 2015–2016 and 2017–2018, which provided the most recent dietary intake data of nationally representative US adults. The NHANES used the US Department of Agriculture Automated Multiple-Pass Method to enhance complete and accurate recall of all foods and beverages consumed in the previous day and reduce respondent burden across all cycles.19 We performed energy adjustment to reduce measurement errors associated with self-reported dietary intake estimates.20 The estimated mean consumption incorporated sampling weights to account for the complex sampling design and ensure national representativeness (Table B, available as a supplement to the online version of this article at http://www.ajph.org). We characterized the optimal intake of each dietary factor based on the consumption level associated with lowest disease risk in meta-analyses of clinical end points, assessed by the Global Burden of Disease 2010.21
Diet‒Cancer Associations
To estimate cancer risks attributable to suboptimal diet, we incorporated both direct diet‒cancer etiologic effects and the body mass index (BMI; defined as weight in kilograms divided by the square of height in meters)‒mediated associations between diet and cancer. We obtained the relative risk estimates for direct diet‒cancer etiologic effects from meta-analysis performed by WCRF/AICR,4,5 which included prospective cohort studies with limited evidence of bias from confounders, where the associations were multivariable adjusted and independent of BMI (Table C and Method B, available as supplements to the online version of this article at http://www.ajph.org). The long-term etiologic effects of dietary factors on BMI were estimated based on multivariable-adjusted pooled analysis from 120 977 US men and women from 3 prospective cohort studies (Table D, available as a supplement to the online version of this article at http://www.ajph.org).7,9 We obtained effects of elevated BMI on cancer from meta-analyses of prospective cohort studies conducted by the International Agency for Research on Cancer6 and WCRF/AICR (Table E, available as supplement to the online version of this article at http://www.ajph.org).8
Cancer Incidence and Survival
We obtained cancer incidence rates for the 15 diet-related cancer types (i.e., colorectal, oral cavity or pharynx, larynx, corpus uteri, kidney, breast, liver, stomach, esophagus, pancreas, prostate, thyroid, gallbladder, ovary, and multiple myeloma) in 2017 from the Centers for Disease Control and Prevention’s National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results program (SEER; Method C and Table F, available as supplements to the online version of this article at http://www.ajph.org).22 To account for underlying trends in cancer incidence for each cancer type, we estimated the average annual percent change in age-adjusted incidence rates from 2008 to 2017, and then applied that to the baseline incidence rates to project future trends in cancer incidence in 2018 and beyond.3 We estimated the annual probability of dying from cancer based on the 5-year relative survival in 2016 obtained from SEER (Method C and Table G, available as supplements to the online version of this article at http://www.ajph.org).
Economic Costs
We obtained direct medical costs for cancer care by age (< 65 and ≥ 65 years), gender, and phase of cancer care (initial, continuing, and end year of life) from National Cancer Institute’s Cancer Prevalence and Cost of Care Projections.3 Indirect societal costs including productivity loss costs and patient time costs associated with cancer care were obtained from published estimates based on the Medical Expenditure Panel Survey data.23,24 All costs were inflated to 2017 US dollars using the Personal Health Care index (Method D and Table H, available as supplements to the online version of this article at http://www.ajph.org).
Uncertainty Analysis
We incorporated uncertainties in each model input parameter using probabilistic sensitivity analyses with 1000 simulations. We derived corresponding 95% uncertainty intervals (UIs) from the 2.5th and 97.5th percentiles of 1000 estimates. We conducted statistical analyses in R, version 3.6.1 (R Foundation, Vienna, Austria).
RESULTS
Among US adults over a lifetime, suboptimal intakes of 7 dietary factors were estimated to cause 3.04 (95% UI = 2.88, 3.20) million new cancer cases, 1.74 (95% UI = 1.65, 1.84) million cancer deaths (Table 1), and $253.69 (95% UI = $241.54, $266.54) billion direct medical costs associated with cancer care (Table 2), accounting for 7.4% of new cancer cases, 7.7% of cancer deaths, and 7.8% of direct medical costs of these 15 cancers in the United States. These diet-attributable cancers also cost $113.89 (95% UI = $108.21, $119.86) billion in productivity losses and $16.10 (95% UI = $15.30, $16.92) billion in patient time costs. Among all diet-attributable cancer burdens, 72.0% of new cancer cases (2.09 million; 95% UI = 1.95, 2.22), 71.3% of cancer deaths (1.24 million; 95% UI = 1.16, 1.31; Table I, available as a supplement to the online version of this article at http://www.ajph.org), and 72.8% of direct medical costs ($184.80 billion; 95% UI = $173.58, $194.92 billion; Table J, available as a supplement to the online version of this article at http://www.ajph.org) were attributable to direct diet‒cancer etiologic effects, and the remainder were attributable to dietary effects mediated through obesity (Table K and Table L, available as supplements to the online version of this article at http://www.ajph.org).
TABLE 1—
Estimated Cancer Burden Attributable to Suboptimal Diet Among US Adults Over a Lifetime by Population Subgroups: 2015‒2018
| New Cancer Cases | Cancer Deaths | |||
| No., Median (95% UI) | No. per 100 000 Population, Median (95% UI) | No., Median (95% UI) | No. per 100 000 Population, Median (95% UI) |
|
| All US adults | 3 040 000 (2 880 000, 3 200 000) | 1 290 (1 230, 1 360) | 1 740 000 (1 650 000, 1840000) | 742 (704, 782) |
| Gender | ||||
| Women | 1 400 000 (1 310 000, 1 490 000) | 1 150 (1 070, 1 220) | 767 000 (714 000, 821 000) | 629 (585, 673) |
| Men | 1 640 000 (1 510 000, 1 760 000) | 1 450 (1 340, 1 560) | 977 000 (906 000, 1 050 000) | 863 (801, 928) |
| Age, y | ||||
| 20–44 | 1 610 000 (1 490 000, 1 750 000) | 1 550 (1 430, 1 680) | 992 000 (919 000, 1 080 000) | 953 (883, 1 030) |
| 45–54 | 564 000 (514 000, 619 000) | 1 390 (1 270, 1 530) | 308 000 (281 000, 338 000) | 761 (694, 835) |
| 55–64 | 503 000 (453 000, 549 000) | 1 180 (1 060, 1 290) | 267 000 (239 000, 292 000) | 624 (560, 682) |
| ≥ 65 | 357 000 (323 000, 394 000) | 747 (675, 824) | 178 000 (161 000, 195 000) | 371 (335, 408) |
| Race/ethnicity | ||||
| Non-Hispanic Black | 377 000 (348 000, 406 000) | 1 400 (1 300, 1 510) | 245 000 (226 000, 266 000) | 915 (842, 992) |
| Hispanic | 485 000 (441 000, 528 000) | 1 330 (1 210, 1 450) | 307 000 (277 000, 337 000) | 840 (757, 923) |
| Other | 265 000 (243 000, 286 000) | 1 130 (1 040, 1 220) | 151 000 (138 000, 166 000) | 645 (587, 707) |
| Education | ||||
| < high school | 398 000 (366 000, 430 000) | 1 320 (1 210, 1 420) | 242 000 (222 000, 263 000) | 801 (733, 869) |
| High school or GED | 766 000 (706 000, 829 000) | 1 360 (1 250, 1 470) | 453 000 (415 000, 493 000) | 802 (735, 873) |
| Some college | 987 000 (911 000, 1 070 000) | 1 330 (1 230, 1 430) | 583 000 (535 000, 633 000) | 784 (720, 852) |
| College or above | 847 000 (775 000, 917 000) | 1 140 (1 050, 1 240) | 495 000 (449 000, 537 000) | 668 (607, 725) |
| Incomea | ||||
| FIPR < 1.3 | 668 000 (614 000, 725 000) | 1 380 (1 270, 1 500) | 408 000 (374 000, 444 000) | 845 (773, 918) |
| FIPR 1.3–2.9 | 910 000 (835 000, 982 000) | 1 310 (1 200, 1 410) | 543 000 (494 000, 591 000) | 780 (710, 850) |
| FIPR ≥ 3.0 | 1 420 000 (1 310 000, 1 520 000) | 1 210 (1 110, 1 300) | 824 000 (757 000, 888 000) | 703 (646, 757) |
| SNAP participation statusb | ||||
| SNAP participants | 596 000 (546 000, 649 000) | 1 460 (1 340, 1 590) | 365 000 (333 000, 400 000) | 894 (815, 980) |
| SNAP-eligible nonparticipants | 285 000 (261 000, 310 000) | 1 310 (1 200, 1 430) | 171 000 (156 000, 188 000) | 788 (717, 867) |
| SNAP-ineligible individuals | 2 090 000 (1 930 000, 2 250 000) | 1 210 (1 120, 1 300) | 1 220 000 (1 130 000, 1 320 000) | 709 (652, 767) |
Note. FIPR = family income to poverty ratio; GED = general equivalency diploma; SNAP = Supplemental Nutrition Assistance Program; UI = uncertainty interval. The federal poverty threshold was according to US Department of Health and Human Services.
The ratio of family income to the federal poverty threshold, adjusting for household size. For reference, the 2017 federal poverty threshold for a family of 4 was $24 600 per year, according to the US Department of Health and Human Services.
SNAP participants are those reporting having ever received household SNAP benefits in the past 12 years in the National Health and Nutrition Examination Survey; SNAP-eligible nonparticipants refer to those who are income eligible for SNAP (FIPR < 1.3) while not reporting SNAP participation in the past 12 years; SNAP-ineligible individuals refer to those with FIPR ≥1.3.
TABLE 2—
Estimated Economic Costs of Cancer Attributable to Suboptimal Diet Among US Adults Over a Lifetime by Population Subgroups: 2015‒2018
| Direct Medical Costs | Productivity Loss Costs | Patient Time Costs | ||||
| Total Costs, Billion $, Median (95% UI) | Costs per 100 000 Population, Million $, Median (95% UI) | Total Costs, Billion $, Median (95% UI) | Costs per 100 000 Population, Million $, Median (95% UI) | Total Costs, Billion $, Median (95% UI) | Costs per 100 000 Population, Million $, Median (95% UI) | |
| All US adults | 253.69 (241.54, 266.54) | 107.88 (102.71, 113.34) | 113.89 (108.21, 119.86) | 48.43 (46.01, 50.97) | 16.1 (15.30, 16.92) | 6.84 (6.50, 7.19) |
| Gender | ||||||
| Women | 108.20 (101.23, 114.82) | 88.65 (82.95, 94.08) | 54.54 (51.00, 58.19) | 44.69 (41.79, 47.68) | 7.93 (7.46, 8.46) | 6.50 (6.11, 6.93) |
| Men | 145.34 (135.40, 156.31) | 128.48 (119.07, 138.18) | 59.28 (55.02, 63.91) | 52.40 (48.64, 56.50) | 8.17 (7.53, 8.8) | 7.22 (6.66, 7.78) |
| Age, y | ||||||
| 20–44 | 121.22 (111.53, 131.50) | 116.42 (107.11, 126.29) | 55.97 (51.26, 60.80) | 53.75 (49.23, 58.39) | 6.83 (6.25, 7.43) | 6.56 (6.01, 7.14) |
| 45–54 | 51.92 (47.11, 56.91) | 128.31 (116.43, 140.67) | 24.27 (22.03, 26.64) | 59.99 (54.45, 65.85) | 3.53 (3.19, 3.88) | 8.71 (7.89, 9.59) |
| 55–64 | 47.20 (42.53, 51.87) | 110.46 (99.53, 121.38) | 20.84 (18.78, 22.91) | 48.77 (43.95, 53.61) | 3.53 (3.17, 3.88) | 8.26 (7.41, 9.09) |
| ≥ 65 | 33.18 (29.74, 36.64) | 69.34 (62.16, 76.58) | 12.91 (11.60, 14.28) | 26.97 (24.25, 29.85) | 2.23 (2.00, 2.47) | 4.65 (4.18, 5.16) |
| Race/ethnicity | ||||||
| Non-Hispanic White | 161.09 (149.36, 173.20) | 108.57 (100.67, 116.74) | 73.92 (68.45, 79.6) | 49.82 (46.13, 53.65) | 10.64 (9.87, 11.45) | 7.17 (6.66, 7.72) |
| Non-Hispanic Black | 32.01 (29.52, 34.39) | 119.33 (110.05, 128.22) | 13.08 (12.16, 14.08) | 48.75 (45.34, 52.49) | 1.78 (1.65, 1.90) | 6.62 (6.16, 7.07) |
| Hispanic | 38.40 (35.08, 41.64) | 105.18 (96.10, 114.07) | 16.67 (15.18, 18.14) | 45.66 (41.58, 49.68) | 2.30 (2.11, 2.48) | 6.29 (5.78, 6.79) |
| Other | 22.09 (20.35, 23.82) | 94.13 (86.73, 101.51) | 10.25 (9.37, 11.02) | 43.70 (39.94, 46.98) | 1.38 (1.27, 1.48) | 5.88 (5.43, 6.29) |
| Education | ||||||
| < high school | 33.54 (30.99, 36.08) | 110.73 (102.32, 119.11) | 14.20 (13.15, 15.27) | 46.89 (43.43, 50.41) | 2.02 (1.88, 2.17) | 6.66 (6.19, 7.16) |
| High school or GED | 64.23 (59.45, 69.28) | 113.77 (105.30, 122.7) | 28.02 (25.90, 30.12) | 49.62 (45.87, 53.36) | 4.00 (3.71, 4.29) | 7.08 (6.57, 7.61) |
| Some college | 82.07 (75.84, 87.92) | 110.39 (102.02, 118.26) | 36.43 (33.78, 39.15) | 49.00 (45.44, 52.67) | 5.12 (4.76, 5.49) | 6.89 (6.41, 7.39) |
| College or above | 71.46 (65.50, 77.37) | 96.48 (88.43, 104.45) | 32.00 (29.38, 34.71) | 43.20 (39.66, 46.86) | 4.47 (4.14, 4.83) | 6.04 (5.59, 6.52) |
| Incomea | ||||||
| FIPR < 1.3 | 54.45 (50.23, 58.60) | 112.68 (103.93, 121.25) | 23.93 (22.08, 26.01) | 49.51 (45.69, 53.81) | 3.32 (3.08, 3.58) | 6.87 (6.36, 7.42) |
| FIPR 1.3–2.9 | 75.22 (68.95, 81.38) | 108.08 (99.07, 116.94) | 32.91 (30.24, 35.65) | 47.30 (43.45, 51.23) | 4.59 (4.25, 4.94) | 6.60 (6.11, 7.10) |
| FIPR ≥ 3.0 | 121.40 (111.64, 129.94) | 103.54 (95.22, 110.83) | 53.86 (49.54, 57.96) | 45.94 (42.25, 49.44) | 7.65 (7.07, 8.21) | 6.52 (6.03, 7.00) |
| SNAP participationb | ||||||
| SNAP participants | 47.86 (43.99, 51.87) | 117.26 (107.76, 127.08) | 21.14 (19.45, 22.97) | 51.78 (47.65, 56.27) | 2.91 (2.68, 3.16) | 7.12 (6.57, 7.73) |
| SNAP-eligible nonparticipants | 23.39 (21.43, 25.43) | 107.55 (98.55, 116.91) | 10.27 (9.38, 11.15) | 47.21 (43.14, 51.28) | 1.42 (1.31, 1.54) | 6.55 (6.02, 7.09) |
| SNAP-ineligible individuals | 177.26 (164.24, 189.96) | 102.7 (95.16, 110.06) | 78.33 (72.51, 84.12) | 45.38 (42.01, 48.74) | 11.06 (10.26, 11.84) | 6.41 (5.95, 6.86) |
Note. FIPR = family income to poverty ratio; GED = general equivalency diploma; SNAP = Supplemental Nutrition Assistance Program; UI = uncertainty interval. The federal poverty threshold was according to US Department of Health and Human Services.
The ratio of family income to the federal poverty threshold, adjusting for household size. For reference, the 2017 federal poverty threshold for a family of 4 was $24 600 per year, according to the US Department of Health and Human Services.
SNAP participants are those reporting having ever received household SNAP benefits in the past 12 years in the National Health and Nutrition Examination Survey; SNAP-eligible nonparticipants refer to those who are income eligible for SNAP (FIPR < 1.3) while not reporting SNAP participation in the past 12 years; SNAP-ineligible individuals refer to those with FIPR ≥ 1.3.
Disparities by Age and Gender
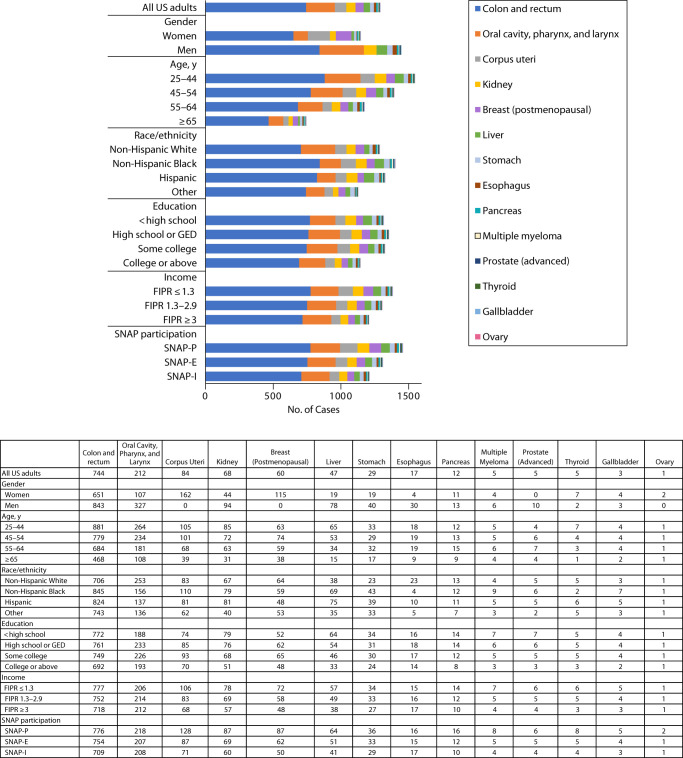
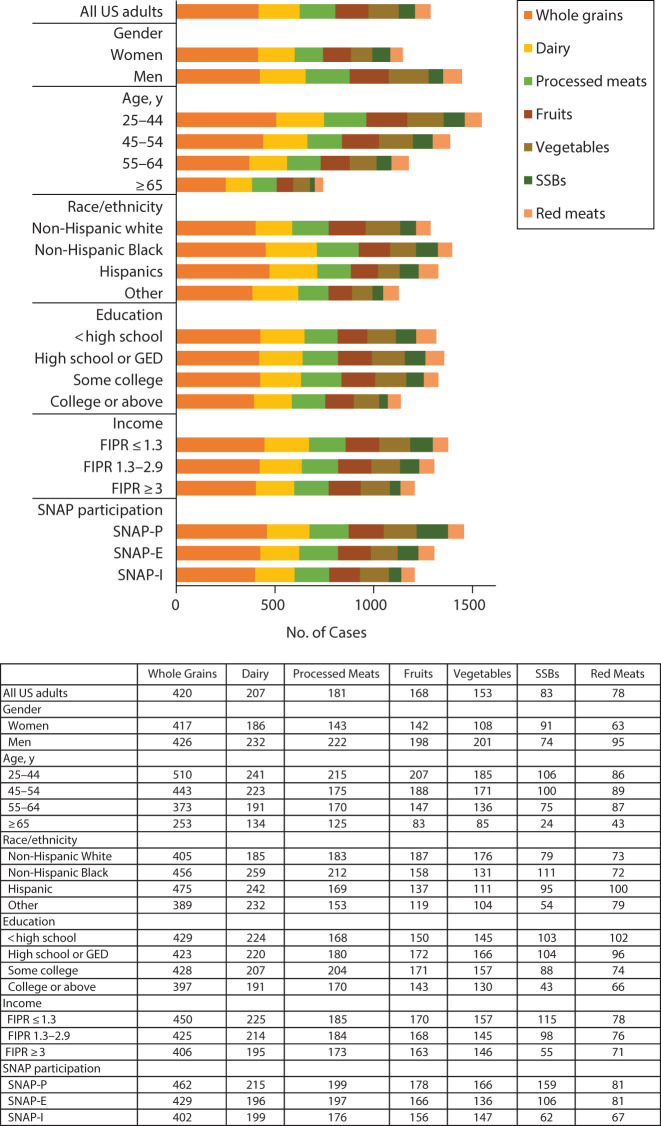
Per 100 000 population, young adults (aged 20–44 years) had a higher number of diet-attributable cancer cases (difference = 803; 95% UI = 656, 952) and cancer deaths (difference = 582; 95% UI = 500, 668) than older adults (≥ 65 years; Table 1). Similar age patterns were observed for all cancer types (Figure 1 and Table M, available as a supplement to the online version of this article at http://www.ajph.org) and all dietary factors (Figure 2 and Table N, available as a supplement to the online version of this article at http://www.ajph.org). Men had a higher number of diet-attributable cancer cases (difference = 300; 95% UI = 169, 434) and cancer deaths (difference = 234; 95% UI = 157, 315) per 100 000 population than women, overall and across cancer types except for female cancers (e.g., female breast, endometrial, and ovary cancers). Men also had a higher diet-attributable cancer burden than women for nearly all dietary factors except for high SSB consumption, which contributed to a higher number of cancer cases in women than in men (11; 95% UI = −10, 43).
FIGURE 1—
Number of Diet-Attributable Cancer Cases by Cancer Types Among Key Population Subgroups of US Adults Over a Lifetime: 2015‒2018
Note. FIPR = family income to poverty ratio; GED = general equivalency diploma; SNAP = Supplemental Nutrition Assistance Program. The federal poverty threshold was according to US Department of Health and Human Services.
FIGURE 2—
Number of Diet-Attributable Cancer Cases by Dietary Factors Among Key Population Subgroups of US Adults Over a Lifetime: 2015‒2018
Note. FIPR = family income to poverty ratio; GED = general equivalency diploma; SNAP = Supplemental Nutrition Assistance Program; SSB = sugar-sweetened beverage. The federal poverty threshold was according to US Department of Health and Human Services.
Disparities by Race/Ethnicity
Non-Hispanic Blacks had more diet-attributable cancer cases (difference = 110; 95% UI = −21, 265) and cancer deaths (214; 95% UI = 126, 309) per 100 000 population than non-Hispanic Whites. Non-Hispanic Blacks also had a higher number of diet-attributable cancer burden for most cancer types, with the largest differences seen for colorectal cancers. However, non-Hispanic Whites had a higher number of diet-attributable cases and deaths for oral cavity, pharynx, or larynx cancers than non-Hispanic Blacks. By dietary factors, non-Hispanic Blacks had a higher number of diet-attributable cancer burden than non-Hispanic Whites for most of the dietary factors, with greater differences attributable to low consumption of dairy and whole grains and high consumption of processed meats and SSBs.
Disparities by Education, Income, and SNAP Status
Compared with those with a college graduate or above level of education, individuals with a lower than college graduate level of education had a higher number of diet-attributable cancer cases (e.g., difference for less than high school vs college graduate = 180; 95% UI = 33, 301) and cancer deaths (132; 95% UI = 46, 219) per 100 000 population. Similar disparities by education were observed for nearly all cancer types, with largest differences seen for colorectal cancer and for nearly all dietary factors with the largest differences attributable to high consumption of SSBs.
Individuals with a low level of family income (FIPR < 1.3) had a higher number of diet-attributable cancer cases (difference = 170; 95% UI = 24, 322) and cancer deaths (difference = 142; 95% UI = 49, 238) per 100 000 population than higher-income individuals (FIPR ≥ 3). Similar disparities were observed across cancer types and dietary factors, with only a few exceptions. Similarly, we observed a higher number of diet-attributable cancer cases and cancer deaths among SNAP participants than eligible nonparticipants (difference = 100; 95% UI = 15, 330) and SNAP-ineligible individuals (difference = 250, 95% UI = 106, 402) for nearly all cancer types and each dietary factor. The largest disparities by family income and SNAP participation status were attributable to high consumption of SSBs and low consumption of whole grains.
Obesity vs Direct Diet‒Cancer Associations
The diet-attributable cancer cases and deaths mediated through obesity were higher among young versus older adults per 100 000 population (difference of cases = 292; 95% UI = 236, 350), women versus men (difference of cases = 128; 95% UI = 78, 182), non-Hispanic Blacks versus non-Hispanic Whites (difference of cases = 95; 95% UI = 21, 160), individuals with low versus higher levels of education (difference of cases = 91; 95% UI = 29, 154) or income (difference of cases = 140; 95% UI = 69, 207), and SNAP participants versus SNAP-ineligible individuals (difference of cases = 162; 95% UI = 87, 242; Table I and Table J). We observed similar disparities for cancer burdens attributable to direct diet‒cancer associations, except that men had a higher direct diet-attributable cancer burden than women (Table K and Table L).
Disparities in Economic Costs
We observed similar disparities for diet-attributable cancer costs for direct medical costs, productivity loss costs, and patient time costs. For example, the direct medical costs of diet-attributable cancers (million per 100 000 population) were higher in young (aged 20‒44 years) versus older adults (aged ≥ 65 years; difference = 46.91; 95% UI = 35.69, 59.03), men versus women (difference = 39.90; 95% UI = 29.47, 50.49), non-Hispanic Blacks versus non-Hispanic Whites (difference = 10.69; 95% UI = −.55, 22.84), individuals with low versus higher levels of education (difference = 14.50; 95% UI = 3.53, 25.29) or income (difference = 9.14; 95% UI = 2.39, 20.68), and SNAP participants versus ineligible individuals (difference = 14.75; 95% UI = 2.20, 25.78). However, middle-aged adults (aged 45‒54 years) had higher diet-attributable costs than either younger (< 45 years) or older (≥ 55 years) adults. These disparity patterns were similarly observed across cancer types and dietary factors (Table O and Table P, available as supplements to the online version of this article at http://www.ajph.org).
DISCUSSION
Based on a nationally representative simulation model, suboptimal diet was estimated to contribute to 3.04 million new cancer cases, 1.74 million cancer deaths, and $254 billion direct medical costs associated with cancer care among US adults over a lifetime. The health and economic burdens of diet-attributable cancers were higher among men, younger adults, racial/ethnic minorities, individuals with lower education and income attainments, and SNAP participants, compared with their counterpart groups. The largest disparities in diet-attributable cancer were associated with high consumption of SSBs and low consumption of whole grains. To our best knowledge, this study is among the first to quantify disparities in both the health and economic burdens of cancer attributable to suboptimal diet in the United States.
Public Health Implications
The higher diet-attributable cancer burdens among non-Hispanic Blacks, individuals with lower education and income attainments, and SNAP participants reflects both a higher cancer risk and a worse diet quality among these population subgroups.10–12 These findings suggest that targeted nutrition interventions among these population subgroups can potentially reduce diet-attributable cancer disparities. In addition, our study revealed that the highest diet‒cancer disparities were attributable to high consumption of SSBs and processed meats and low consumption of whole grains and dairy. These could be priority dietary targets for behavior change and policy strategies to reduce cancer disparities in the United States. Policy options may include expanding SNAP to include financial incentives for purchasing whole grains, fruits, and vegetables and disincentives to discourage the consumption of SSBs and processed meats.25,26 Other relevant policy actions may include improving the availability, affordability, and accessibility of healthy foods in low-income and racial/ethnic minority communities, schools, and workplaces.27
The gender difference in diet-attributable cancer burdens is also worth attention. The overall higher diet-attributable cancer burdens in men than women may reflect a worse diet quality in men than women.11,13 For example, the Healthy Eating Index 2015, a diet quality index that measures adherence to the 2015–2020 Dietary Guidelines for Americans, was 5% (3 percentage points) lower in men than women.13 However, women had a higher diet-attributable cancer burden mediated through obesity than men. It is possible that excessive body weight has a larger impact on female cancers. In accordance with this finding, our results also revealed that high SSB consumption contributed to more cancer cases in women than in men. The higher diet-related cancer burdens estimated among younger than older adults were primarily attributable to the longer length of follow-up of younger adults in a closed cohort.
Consumption of SSBs contributed to the largest diet-attributable cancer disparities in the United States, by age, race, and socioeconomic status. Although SSB consumption had declined by 28% since 1999 among US adults, level of consumption remains high, especially among young adults, racial/ethnic minorities, and socioeconomically disadvantaged groups.12 Reducing SSB consumption through relevant policy actions, including taxes, warning labels, or Nutrition Facts Panel labeling of added sugars28,29 could be effective strategies for reducing diet-attributable cancer disparities. Although restricting SSB purchases for SNAP participants has been debated,30 a combination of financial disincentives for SSBs and other less healthful foods plus incentives for a range of healthful foods may help reduce disparities while still preserving choice.31
Low whole grain consumption also contributed to substantial diet‒cancer disparities in the United States. Despite modest recent increase in whole grain consumption, current levels of 1 serving a day remain far below the recommended intake of 3 servings per day.11 In 2015 to 2016, US adults consumed only 2.7% of calories from whole grains, compared with 15.9% of calories from refined grains.13 Potential barriers for increasing whole grain consumption include the lack of public awareness on health benefits of whole grains, lack of knowledge to identify whole grain products, and absence of standardized definitions and labeling to increase awareness and healthier choices.32 Efforts are needed to address these challenges,9,32 including incorporating additional financial incentives for healthy whole grain products (rather than only fruits and vegetables) in SNAP.31
By cancer type, optimizing dietary intake could be particularly important for reducing disparities related to colorectal cancer. Colorectal cancer is the third most diagnosed cancer among US men and women1 and is associated with the largest number of diet-attributable cases.9 Colorectal cancer disproportionally affects men, non-Hispanic Blacks, and socioeconomically disadvantaged groups,14,15 which runs parallel with the higher colorectal cancer burden attributable to diet observed in this study.
Strengths and Limitations
Our study had several strengths. We used a probabilistic cohort state-transition model, which simulates the transitions among various cancer-related health states along with aging, allowing us to project the health and economic burdens of cancer attributable to diet over a lifetime. Our model incorporated national representative estimates for recent dietary intakes of US adults, national data for cancer incidence and survival, and multivariate-adjusted etiology effects for diet‒cancer associations from meta-analysis of cohort studies. In addition to estimating direct diet‒ cancer associations independent of obesity, our model further incorporated obesity-mediated cancer risks, allowing us to estimate diet-attributable cancer burdens attributable to obesity-mediated pathways. Our model also accounted for the uncertainty of dietary intake and cancer incidence, allowing estimation of the lower and upper bounds of the plausible effects.
Potential limitations should also be considered. First, self-reported dietary assessment is subject to measurement error. Yet the NHANES is the only nationally representative survey of Americans’ diet; dietary intake data collected in NHANES are often used to evaluate dietary intake patterns of US adults and children.11 In addition, the use of multiple-pass method19 and energy adjustments reduce measurement error.20
Second, we did not incorporate secular trends in diet over time or changes in diet across the life course. If disparities in dietary intake patterns persist or worsen in future years or as people age, the estimated disparities in diet-associated cancer burdens are likely to sustain to later years or become more profound. In addition, the current estimates did not consider the impact of early life diet on cancer outcomes and could have underestimated cancer burdens attributable to suboptimal diet.
Third, the projected rates of cancer incidence and mortality in the current model may not fully capture the influence of Affordable Care Act and other changing policies and factors on cancer outcomes. Disparities in diet-associated health and economic burdens of cancer may decline in future years if the insurance expansion under the Affordable Care Act is likely to reduce cancer disparities in the United States.33
Fourth, cancer could lead to substantial psychological burdens for the patients and their families. Our model did not include the psychological burden of cancer because of the lack of well-accepted methods to quantify it;34 the diet-associated cancer burdens could be underestimated.
Fifth, we assumed a 5-year induction period between changes in current dietary intakes to optimal intakes and cancer risks, based on limited empiric evidence of the induction time between diet and various cancers.35 Longer or shorter induction periods could partly alter our findings.
Conclusions
Suboptimal diet contributes to a substantial cancer burden in racial/ethnic minorities, socioeconomically disadvantaged groups, SNAP participants, men, and young adults. High consumption of SSBs and low consumption of whole grains are 2 leading dietary targets for prevention policies to reduce diet-attributable cancer disparities in the United States. These findings underscore the importance of developing and implementing targeted food and nutrition strategies among key population subgroups to reduce cancer disparities in the United States.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health, National Institute on Minority Health and Health Disparities grant 1R01MD011501.
Note. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
PUBLICATION INFORMATION
Full Citation: Wang L, Du M, Cudhea F, et al. Disparities in health and economic burdens of cancer attributable to suboptimal diet in the United States, 2015‒2018. Am J Public Health.
Acceptance Date: July 2, 2021.
CONFLICTS OF INTEREST
The authors declare no competing interests.
HUMAN PARTICIPANT PROTECTION
The study is exempt for ethical review and waived for consent because it used publicly available data with no personal identifiable information.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. . 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.American Association for Cancer Research. 2020. https://cancerprogressreport.org/Pages/cpr19-contents.aspx
- 3. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010‒2020. J Natl Cancer Inst. . 2011;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Cancer Research Fund International/American Institute for Cancer Research. https://www.wcrf.org/dietandcancer/a-summary-of-the-third-expert-report
- 5.World Cancer Research Fund/American Institute for Cancer Research. 2013. https://www.wcrf.org/sites/default/files/mouth-pharynx-larynx-cancer-protocol.pdf
- 6. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. . 2016;375(8):794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. . 2011;364(25):2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Cancer Research Fund/American Institute for Cancer Research. 2018. https://www.wcrf.org/dietandcancer/body-fatness-and-weight-gain
- 9. Zhang FF, Cudhea F, Shan Z, et al. Preventable cancer burden associated with poor diet in the United States. JNCI Cancer Spectr. . 2019;3(2):pkz034. doi: 10.1093/jncics/pkz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Webb M, Fahimi S, Singh GM, et al. Cost effectiveness of a government supported policy strategy to decrease sodium intake: global analysis across 183 nations. BMJ. . 2017;356:i6699. doi: 10.1136/bmj.i6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rehm CD, Penalvo JL, Afshin A, Mozaffarian D. Dietary intake among US adults, 1999‒2012. JAMA. . 2016;315(23):2542–2553. doi: 10.1001/jama.2016.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang FF, Liu J, Rehm CD, Wilde P, Mande JR, Mozaffarian D. Trends and disparities in diet quality among US adults by Supplemental Nutrition Assistance Program participation status. JAMA Netw Open. . 2018;1(2):e180237. doi: 10.1001/jamanetworkopen.2018.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shan Z, Rehm CD, Rogers G, et al. Trends in dietary carbohydrate, protein, and fat intake and diet quality among US adults, 1999‒2016. JAMA. . 2019;322(12):1178–1187. doi: 10.1001/jama.2019.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jackson CS, Oman M, Patel AM, Vega KJ. Health disparities in colorectal cancer among racial and ethnic minorities in the United States. J Gastrointest Oncol. . 2016;7(suppl 1):S32–S43. doi: 10.3978/j.issn.2078-6891.2015.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doubeni CA, Laiyemo AO, Major JM, et al. Socioeconomic status and the risk of colorectal cancer. Cancer. . 2012;118(14):3636–3644. doi: 10.1002/cncr.26677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim DD, Wilde PE, Michaud DS, et al. Cost effectiveness of nutrition policies on processed meat: implications for cancer burden in the US. Am J Prev Med. . 2019;57(5):e143–e152. doi: 10.1016/j.amepre.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. . 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 18.National Health and Nutrition Examination Survey. 2020. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/DEMO_I.htm
- 19.Moshfegh AJ, Rhodes DG, Baer DJ, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88(2):324–332. doi: 10.1093/ajcn/88.2.324. [DOI] [PubMed] [Google Scholar]
- 20.Willett W. Nutritional Epidemiology. Oxford Scholarship Online; 2012. Implications of total energy intake for epidemiologic analyses. [DOI] [Google Scholar]
- 21.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990‒2010: a systematic analysis for the Global Burden of Disease Study 2010 [errata in Lancet. 2013;381(9874):1276 and Lancet. 2013;381(9867):628]. Lancet. 2012. 38098592224–2260. 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services, Centers for Disease Control and Prevention, National Cancer Institute. 2020. https://www.cdc.gov/cancer/uscs/public-use
- 23. Zheng Z, Yabroff KR, Guy GP., Jr et al. Annual medical expenditure and productivity loss among colorectal, female breast, and prostate cancer survivors in the United States. J Natl Cancer Inst. . 2015;108(5):djv382. doi: 10.1093/jnci/djv382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yabroff KR, Guy GP., Jr Ekwueme DU. et al. Annual patient time costs associated with medical care among cancer survivors in the United States. Med Care. . 2014;52(7):594–601. doi: 10.1097/MLR.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barnhill A. Impact and ethics of excluding sweetened beverages from the SNAP program. Am J Public Health. . 2011;101(11):2037–2043. doi: 10.2105/AJPH.2011.300225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blumenthal SJ, Hoffnagle EE, Leung CW, et al. Strategies to improve the dietary quality of Supplemental Nutrition Assistance Program (SNAP) beneficiaries: an assessment of stakeholder opinions. Public Health Nutr. 2014;17(12):2824–2833. doi: 10.1017/S1368980013002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mande JWW, Auerbach J, Bleich S, et al. 2020. https://sites.tufts.edu/foodnutritionandhealth2019
- 28. Wilde P, Huang Y, Sy S, et al. Cost-effectiveness of a US national sugar-sweetened beverage tax with a multistakeholder approach: who pays and who benefits. Am J Public Health. . 2019;109(2):276–284. doi: 10.2105/AJPH.2018.304803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang Y, Kypridemos C, Liu J, et al. Cost-effectiveness of the US Food and Drug Administration added sugar labeling policy for improving diet and health. Circulation. . 2019;139(23):2613–2624. doi: 10.1161/CIRCULATIONAHA.118.036751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paarlberg R, Mozaffarian D, Micha R, Chelius C. Keeping soda in SNAP: understanding the other Iron Triangle. Society. . 2018;55(4):308–317. doi: 10.1007/s12115-018-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Afshin A, Penalvo JL, Del Gobbo L, et al. The prospective impact of food pricing on improving dietary consumption: a systematic review and meta-analysis. PLoS One. . 2017;12(3):e0172277. doi: 10.1371/journal.pone.0172277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilde P, Pomeranz JL, Lizewski LJ, Zhang FF. Consumer confusion about wholegrain content and healthfulness in product labels: a discrete choice experiment and comprehension assessment. Public Health Nutr. . 2020;23(18):3324–3331. doi: 10.1017/S1368980020001688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim U, Koroukian S, Statler A, Rose J. The effect of Medicaid expansion among adults from low‐income communities on stage at diagnosis in those with screening‐amenable cancers. Cancer. . 2020;126(18):4209–4219. doi: 10.1002/cncr.32895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Essue BM, Iragorri N, Fitzgerald N, de Oliveira C. The psychosocial cost burden of cancer: a systematic literature review. Psychooncology. . 2020;29(11):1746–1760. doi: 10.1002/pon.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bernstein AM, Song M, Zhang X, et al. Processed and unprocessed red meat and risk of colorectal cancer: analysis by tumor location and modification by time. PLoS One. . 2015;10(8):e0135959. doi: 10.1371/journal.pone.0135959. [DOI] [PMC free article] [PubMed] [Google Scholar]