Figure 1.
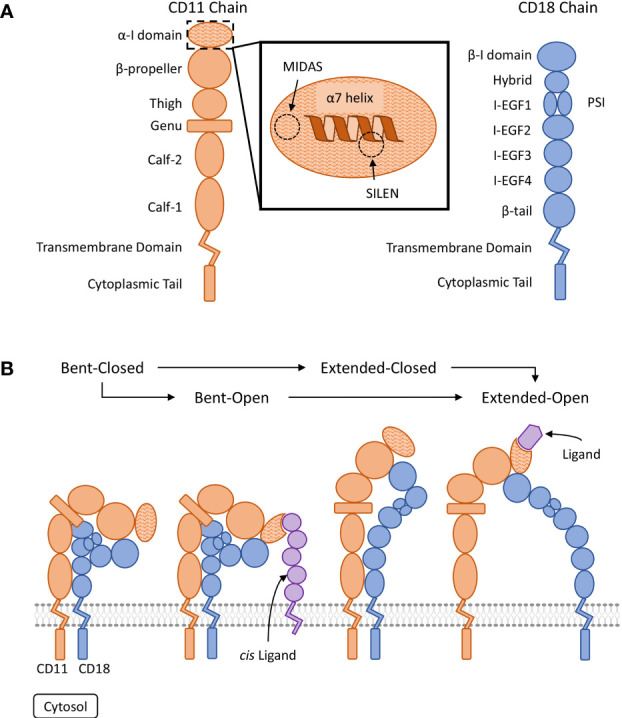
Visual representation of β2 integrin structure and conserved regulatory conformations. (A) Organization of the domains composing the CD11 and CD18 chains (15, 16). The ligand binding α-I domain is highlighted by a hatched pattern. The metal ion-dependant adhesion site (MIDAS) and the socket for isoleucine (SILEN) motifs are located within the α-I domain (16, 17). The SILEN motif interacts with an invariant isoleucine located in the α7 helix to maintain the inactive conformation (17). (B) Representation of the β2 integrin regulatory conformations. The bent-closed inactive conformation predominates under basal conditions (1). Stimulation can activate the integrin and induce the extended-closed conformation. Additional stimulation and the binding of a divalent metal ion to the MIDAS motif, can induce the extended-open conformation. The bent-open conformation is stabilized by binding a cis ligand and may provide an alternative activation pathway to the extended-open conformation (18–20). The extended-open conformation is characterized by the separation of the cytosolic tails in additional to local conformational changes within the α-I domain, including shifting of the α7 helix (14–16, 21, 22).
