Abstract
Background:
Per- and polyfluoroalkyl substances (PFAS) are widely used chemicals, some of which have been linked to type 2 diabetes. We tested whether PFAS concentrations were cross-sectionally associated with metabolites previously shown to predict incident type 2 diabetes using the Diabetes Prevention Program (DPP), a trial of individuals at high risk of type 2 diabetes.
Methods:
We evaluated 691 participants enrolled in the DPP with baseline measures of 10 PFAS (including total perfluorooctanesulfonic acid (PFOS), total perfluorooctanoic acid (PFOA), and Sb-PFOA [branched isomers of PFOA]) and 77 metabolites. We used log2-transformed PFAS concentrations as exposures and standardized metabolite concentrations as outcomes in linear regression models adjusted for age, sex, race/ethnicity, use of anti-hyperlipidemic or triglyceride-lowering medication, income, years of education, marital status, smoking, and family history of diabetes, with Benjamini-Hochberg linear step-up false discovery rate correction.
Results:
Sb-PFOA was associated with the largest number of tested metabolites (29 of 77). Each doubling in Sb-PFOA was associated with higher leucine (β=0.07 [95%CI: 0.02, 0.11] SD) and lower glycine (−0.08 [95%CI: −0.03, −0.13] SD). Each doubling of either total PFOA or n-PFOA was associated with −0.13 [95%CI: −0.04, −0.22] SD lower glycine. PFOA and Sb-PFOA were positively associated with multiple triacylglycerols and diacylglycerols, and total PFOS, total PFOA, and Sb-PFOA were positively associated with phosphatidylethanolamines.
Conclusions:
PFAS concentrations are associated with metabolites linked to type 2 diabetes (particularly amino acid, glycerolipid and glycerophospholipid pathways). Further prospective research is needed to test whether these metabolites mediate associations of PFAS and type 2 diabetes.
Keywords: PFAS, metabolomics, type 2 diabetes
Introduction
Per- and polyfluoroalkyl substances (PFAS) are industrial chemicals that have been manufactured in the United States and worldwide for decades [1]. They are used in consumer products, including cooking equipment, food packaging, and water- and stain-resistant fabric treatments [2–4]. Due to PFAS’s widespread use, nearly all Americans have detectable concentrations of PFAS in their blood [5].
Some studies suggest that certain PFAS may elevate risk of type 2 diabetes [6–8]. For example, a prospective case-control study nested in the Nurses’ Health Study II reported that concentrations of both perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) were associated with higher odds of developing type 2 diabetes during follow-up [6]. Two studies in the Diabetes Prevention Program (DPP), a population at high risk of developing type 2 diabetes, reported similar findings. Specifically, over a median of 3 years of follow-up, concentrations of branched isomers of PFOA (Sb-PFOA) were associated with increased risk of developing type 2 diabetes [7], and this association remained significant after extending the follow-up to ~9 years (in the placebo group only) [8]. However, not all prospective studies reported positive findings [9, 10].
Certain metabolic pathways are altered before the onset of overt diabetes, and changes in these metabolites may predict which individuals are at high risk of developing diabetes. For example, higher concentrations of branched-chain amino acids (BCAAs: isoleucine, leucine, and valine), higher concentrations of aromatic amino acids (AAAs: tyrosine and phenylalanine), and lower concentrations of glycine and glutamine have been associated with developing type 2 diabetes in multiple epidemiological studies [11]. In a recent cohort, phosphatidylcholines were linked to lower risk of type 2 diabetes, while phosphatidylethanolamines were associated with increased risk [12]. Studies in the DPP have identified additional plasma metabolites associated with type 2 diabetes. A nested case-control analysis within DPP participants identified four metabolites associated with developing diabetes (methionine sulfoxide, propionylcarnitine, betaine, and serine) [13], and a larger cohort analysis reported prospective associations with 69 metabolites across all treatment groups, in addition to validating associations with betaine and methionine sulfoxide but not propionylcarnitine or serine [14]. The biological mechanism connecting PFAS exposure to type 2 diabetes in humans is not clear, but preliminary evidence from animal and in vitro studies suggests that PFAS may disrupt specific metabolic pathways due to the structural similarities between PFAS and free fatty acids. For example, PFAS may interfere with lipid metabolism by activating PPAR-α [15, 16], may increase oxidative stress by upregulating fatty acid oxidation pathways [17], and/or might alter amino acid metabolic pathways [18], all of which can lead to insulin resistance and eventual onset of diabetes.
To understand how PFAS exposure might contribute to an increased risk of type 2 diabetes, a first step is to explore these potential mechanisms by identifying which metabolites associated with type 2 diabetes are also associated with exposure to PFAS. In this study, we used DPP data to evaluate cross-sectional associations of 10 PFAS and 77 metabolites previously shown to have associations with developing type 2 diabetes in DPP participants [14]. Our primary hypothesis was that some PFAS would be associated with BCAAs, AAAs, glycine, and glutamine; we also hypothesized that PFAS would be associated with other metabolites linked to developing type 2 diabetes in the DPP.
Methods
The DPP is a multi-center randomized clinical trial of individuals at high risk of type 2 diabetes. Participants were eligible for recruitment in 1996–1999 if they were at least 25 years old, had a body mass index (BMI) of at least 24 kg/m2 (22 kg/m2 for Asian-Americans), and had impaired glucose tolerance and a fasting plasma glucose of 5.3–6.9 mmol/L ( ≤6.9 mmol/L for American Indians). Individuals were excluded if they had a hospitalization for heart disease within 6 months, heart failure, heart block, uncontrolled hypertension, or certain cancers; if they had other conditions that might limit participation; or if they were using medications that could impair glucose tolerance. All participants provided written informed consent in accordance with institutional review board requirements of the recruiting center [19].
We measured plasma PFAS concentrations in available fasting baseline plasma samples from participants in two of the three treatment arms in the DPP trial (lifestyle and placebo arms; total available n=957). Centers for Disease Control and Prevention (CDC) scientists quantified plasma concentrations of 13 PFAS using a modification of the online solid-phase extraction-high-performance liquid chromatography coupled to isotope dilution-tandem mass spectrometry (MS) [20]. The quantified PFAS were: total PFOS, n-PFOS (linear PFOS), Sm-PFOS (a monomethyl branched isomer of PFOS), Sm2-PFOS (another monomethyl branched isomer of PFOS), total PFOA, n-PFOA (linear PFOA), Sb-PFOA, perfluorononanoic acid (PFNA), perfluorohexane sulfonic acid (PFHxS), 2-(N-ethyl-perfluorooctane sulfonamido) acetic acid (EtFOSAA), 2-(N-methyl-perfluorooctane sulfonamido) acetic acid (MeFOSAA), perfluorodecanoic acid (PFDeA), and perfluorooctanesulfonamide (PFOSA). Total PFOS is the sum of n-PFOS, Sm-PFOS, and Sm2-PFOS; total PFOA is the sum of n-PFOA and Sb-PFOA. The limit of detection (LOD) for all PFAS was 0.1 ng/mL. We imputed values below the LOD using to avoid left-truncating the exposure distribution by excluding low values from analysis (0–16.7% of values were imputed; Table 1) [21]. All PFAS except Sm2-PFOS, PFDeA, and PFOSA were detected in over 60% of samples (Table 1); we excluded Sm2-PFOS, PFDeA, and PFOSA from analysis. Analysis of deidentified samples at the CDC laboratory did not constitute engagement in human subjects research.
Table 1.
Descriptive statistics of PFAS (ng/mL) in 691 DPP participants included in this study
| PFAS | % <LOD | Geometric mean | 5% | 25% | 50% | 75% | 95% |
|---|---|---|---|---|---|---|---|
| Total PFOS | -- | 26.1 | 9.2 | 17.3 | 26.6 | 40.3 | 72.4 |
| n-PFOS | 0% | 18.3 | 6.2 | 11.7 | 18.3 | 28.5 | 53.0 |
| Sm-PFOS | 0% | 7.3 | 2.4 | 4.8 | 7.6 | 11.3 | 20.2 |
| Sm2-PFOS | 60.4% | 0.1 | -- | -- | -- | 0.3 | 0.6 |
| Total PFOA | -- | 4.9 | 2.0 | 3.6 | 5.0 | 6.8 | 12.0 |
| n-PFOA | 0% | 4.4 | 1.8 | 3.2 | 4.4 | 6.0 | 10.0 |
| Sb-PFOA | 16.7% | 0.4 | -- | 0.3 | 0.5 | 0.9 | 1.7 |
| PFHxS | 0.1% | 2.4 | 0.7 | 1.5 | 2.3 | 3.8 | 10.7 |
| PFNA | 7.1% | 0.5 | -- | 0.4 | 0.6 | 0.8 | 1.9 |
| EtFOSAA | 3.5% | 1.1 | 0.2 | 0.6 | 1.2 | 2.1 | 5.7 |
| MeFOSAA | 2.9% | 0.9 | 0.3 | 0.5 | 1.0 | 1.7 | 3.3 |
| PFDeA | 76.6% | 0.1 | -- | -- | -- | -- | 0.5 |
| PFOSA | 62.0% | 0.1 | -- | -- | -- | 0.3 | 0.9 |
LOD was 0.1 ng/mL for all PFAS.
Total PFOS = n-PFOS + Sm-PFOS + Sm2-PFOS. Total PFOA = n-PFOA + Sb-PFOA. Abbreviations: LOD, limit of detection; EtFOSAA, 2-(N-ethyl-perfluorooctane sulfonamido) acetic acid; MeFOSAA, 2-(N-methyl-perfluorooctane sulfonamido) acetic acid; n-PFOA, linear PFOA; n-PFOS, linear PFOS; PFDeA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid; PFOSA, perfluorooctanesulfonamide; Sb-PFOA, branched isomers of PFOA; Sm-PFOS, monomethyl branched isomer of PFOS; Sm2-PFOS, monomethyl branched isomer of PFOS.
Fasting plasma samples were collected at baseline, aliquoted, and stored at −80°C until analysis [19]. As part of a previous case-control study, DPP investigators thawed 2,015 of the frozen baseline samples (1,008 individuals who developed cancer or cardiovascular disease after baseline, and 1,007 individuals without these outcomes selected at random) and quantified metabolite concentrations. Analytic methods have been described previously [13, 14]. Briefly, the investigators quantified metabolite concentrations using three platforms: (1) hydrophilic interaction chromatography (Waters; Milford, MA) coupled to a Q Exactive MS in positive ion mode (Thermo Fisher Scientific, Waltham, MA) to quantify amino acids and amines; (2) C8 chromatography (Waters; Milford, MA) coupled to a Q Exactive MS in positive ion mode to quantify lipids; (3) amide chromatography (Waters; Milford, MA) coupled to an Agilent 6490 triple quadrupole MS (Agilent Technologies, Santa Clara, CA) using negative ion mode electrospray ionization to quantify organic acids [14]. Investigators excluded metabolites from analysis if they were missing in more than 10% of samples or had assay coefficient of variation ≥25% (9% of metabolites; n=34 metabolites).
We then selected metabolites associated with incident type 2 diabetes, focusing mainly on metabolites found to predict type 2 diabetes in the DPP [11, 13, 14]. We selected 77 metabolites: seven whose pooled effects, calculated in a recent meta-analysis of prospective studies, indicated that they were statistically significantly associated with incident type 2 diabetes at the alpha < 0.05 level (positive associations: isoleucine, leucine, valine, tyrosine, and phenylalanine; inverse associations: glycine, glutamine) [11]; two that were statistically significantly associated (at the alpha < 0.05 level) with incident type 2 diabetes in a case-control study in the DPP [13] and were validated in a DPP cohort study (positive association: methionine sulfoxide; inverse association: betaine); and 68 additional metabolites that were statistically significantly associated (at the alpha < 0.05 level) with incident type 2 diabetes in a DPP cohort study (positive associations: dimethylglycine, cotinine, C5 carnitine, C5-DC carnitine, glucose/fructose/galactose [composite measure due to identical mass of these metabolites], 11 diacylglycerols [DAGs], 27 triacylglycerols [TAGs], and 8 phosphatidylethanolamines [PEs]; and inverse associations: 1,5 AG/1-deoxyglucose, C18:1 carnitine, C16:0 ceramide (d18:1), C18:2 ceramide, cytosine, bilirubin, 6 plasmalogens, 5 sphingomyelins [SMs]) [14]. Metabolite labels are formatted as total number of carbon atoms: total number of double bonds. Supplemental Table S1 lists all abbreviations, including metabolite notation. A complete list of the 77 metabolites can be found in Supplemental Table S2.
Statistical analysis
We examined associations of baseline PFAS concentrations and baseline concentrations of metabolites previously associated with incident type 2 diabetes. We log2-transformed PFAS concentrations and standardized metabolite concentrations to create Z-scores. We used complete case analysis (1%−7.8% of observations were missing, depending on metabolite). Effect estimates represent a difference in standard deviation (SD) in metabolite concentrations per doubling of PFAS concentrations. In addition to testing individual metabolites, we grouped metabolites using chemical class and tested the groups. We created each group by summing the standardized metabolites and dividing by the number of summed metabolites (to maintain a similar scale across groups with different numbers of metabolites). The groups were: Σamino acids (n=10 metabolites) and 3 subgroups (ΣBCAAs [n=3], Σ AAAs [n=2], and Σglycine,glutamine [n=2]); Σglycerolipids (n=38) and two subgroups (Σdiacylglycerols [n=11] and Σtriacylglycerols [n=27]); Σglycerophospholipids (n=14) and two subgroups (Σplasmalogens [n=6] and Σphosphatidylethanolamines [n=8]; Σcarnitines (n=3); and Σsphingolipids (n=5).
The analytic sample (n=691) of individuals with both PFAS and metabolite measurements is composed of the overlap of two sub-samples of the entire DPP cohort (n=3,234): participants with metabolite measurements (n=2015) and those in the lifestyle or placebo DPP trial arms with PFAS measurements (n=957). The sub-sample with metabolite measurements is a previous cancer and cardiovascular disease case-control study; therefore, approximately half the individuals in this analytic sample were selected as incident cancer or cardiovascular disease cases, making our sample unrepresentative of the DPP population. Because higher concentrations of some PFAS have been associated with developing type 2 diabetes [8], it is likely that individuals with higher PFAS concentrations are also overrepresented in the overlap between the two sub-samples. To correct for potential selection bias arising from the way we selected this sample [22], we decided a priori to weight analyses using inverse probability weights. We weighted individuals by cancer or cardiovascular disease case status; among both cancer/cardiovascular disease cases and controls, weights were calculated as one over the probability of having the metabolites measured, multiplied by one over the probability of being in the analytic sample given that they have the metabolites measured.
Participants provided demographic information and personal and family medical history in a baseline interview. Trained clinic staff measured weight and height [19], which we used to calculate BMI (weight (kg)/height(m)2). We adjusted all models for age, sex, race/ethnicity (black, white, or Hispanic/other), cholesterol-lowering medication, triglyceride-lowering medication, income (<$35000, $35–50000, $50–75000, >$75000/year), years of education (<13, 13–16, ≥17), marital status (married, divorced/separated/widowed, never married), smoking (current, former, never), and family history of type 2 diabetes (parent, child, or sibling). We chose these confounders a priori using directed acyclic graphs based on factors previously shown to predict PFAS concentrations and type 2 diabetes [8, 23, 24]. BMI and waist circumference may be confounders, but because PFAS are obesogenic [25], BMI and waist circumference may also mediate the association of PFAS and metabolites; we did not include these covariates in main models to avoid adjusting for a mediator.
We used multivariable linear regression to evaluate associations of each PFAS concentration with each metabolite concentration, adjusted for covariates. We accounted for multiple comparisons using Benjamini-Hochberg false discovery rate (FDR) correction [26] within each set of 77 tests for each PFAS. The FDR correction is a step-up procedure that controls the proportion of falsely rejected hypotheses, assuming independence or positive regression dependency among the test statistics [27]. Positive regression dependency in this context essentially means that the probability of correctly rejecting the null hypothesis increases monotonically as each unadjusted p-value gets smaller. We considered corrected p-values <0.05 to be statistically significant, meaning that we hold the proportion of falsely rejected tests at 5%. We conducted analyses in SAS 9.4 (SAS Institute Inc., Cary, NC).
Sensitivity analysis
Because BMI and waist circumference may be mediators, confounders, or both, we repeated the main analyses adjusting for BMI and waist circumference. To account for sex-specific confounders, we repeated the main analyses restricted to women and adjusting for parity and menopausal status. To relax the assumption of a linear association, we repeated the main analyses with each PFAS divided into quartiles. To adjust for potential confounding from other PFAS, we repeated the main analyses including all PFAS except total PFOS and total PFOA in a single model (we excluded total PFOS and total PFOA because they are sums of other PFAS isomers in the models). We evaluated multicollinearity in the all-PFAS model using the condition index and variance inflation factors.
Results
Our analytic population of 691 participants was mostly non-Hispanic white (60%), married or living with a partner (67%), and never smokers (55%); about three-quarters had completed ≥12 years of education (Table 2). More than two-thirds of participants had a family history of type 2 diabetes (Table 2). Most female participants were postmenopausal (61%) and parous (82%) (Table 2). Weighting to correct for potential selection bias had little impact on the distribution of demographic characteristics; unweighted descriptive statistics are shown in Supplemental Table S3.
Table 2:
Study demographics at baseline (weighted to correct for potential selection bias)
| Characteristic | Overall (n=691 unweighted) | Men (n=224 unweighted) | Women (n=467 unweighted) |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
|
|
|||
| Age (years) | 53.2 (21.8) | 56.3 (23.7) | 51.8 (20.1) |
| Body Mass Index (BMI, kg/m 2 ) | 33.5 (13.8) | 32.0 (12.3) | 34.2 (14.2) |
| n1 (%) | n (%) | n (%) | |
|
|
|||
| Race/Ethnicity | |||
| Non-Hispanic White | 416 (60.2) | 143 (63.8) | 274 (58.6) |
| Non-Hispanic Black | 131 (19.0) | 32 (14.5) | 98 (21.0) |
| Hispanic | 117 (16.9) | 39(17.5) | 77 (16.6) |
| Other | 27 (4.0) | 10 (4.3) | 18 (3.8) |
| Education (years) | |||
| ≤12 | 192 (27.9) | 51 (22.9) | 140 (30.1) |
| 13–16 | 325 (47.0) | 110 (49.0) | 215 (46.1) |
| ≥17 | 174 (25.1) | 63 (28.1) | 111 (23.8) |
| Annual Income | |||
| <$35,000 | 212 (30.6) | 53 (23.7) | 157 (33.7) |
| $35,000 - <50,000 | 131 (19.0) | 43 (19.1) | 89 (19.0) |
| $50,000 - <$75,000 | 134 (19.4) | 47 (20.9) | 87 (18.7) |
| ≥$75,000 | 155 (22.5) | 59 (26.3) | 97 (20.8) |
| Refuse to say | 59 (8.5) | 22 (10.0) | 37 (7.9) |
| Marital Status | |||
| Never married | 75 (10.9) | 26 (11.6) | 49 (10.5) |
| Married or living together | 464 (67.2) | 170 (76.1) | 295 (63.3) |
| Separated, divorced, or widowed | 152 (21.9) | 28 (12.4) | 122 (26.2) |
| Smoking status | |||
| Never smoker | 378 (54.8) | 97 (43.3) | 280 (59.9) |
| Current smoker | 45 (6.6) | 10 (4.3) | 36 (7.6) |
| Former smoker | 267 (38.7) | 117 (52.4) | 152 (32.5) |
| Family history of diabetes | 468 (67.7) | 142 (63.2) | 326 (69.7) |
| Post-menopausal | -- | -- | 286 (61.3) |
| Nulliparous | -- | -- | 87 (18.5) |
Ns scaled to the unweighted total and then rounded to nearest integer. Ns may not sum to totals due to rounding error.
Concentrations of most PFAS were moderately correlated (Supplemental Table S4). N-PFOS and Sm-PFOS were highly correlated with total PFOS, because they partially compose the sum of total PFOS; total PFOA and n-PFOA were also highly correlated (ρ=0.99), though total PFOA and Sb-PFOA were less correlated (ρ=0.81).
The number of statistically significant associations with metabolites varied across PFAS. Total PFOS, n-PFOS, and Sm-PFOS were significantly associated with 17, 17, and 10 metabolites, respectively (Supplemental Figures S1–S3). After FDR correction, total PFOS, n-PFOS, and Sm-PFOS were associated with 8, 6, and 7 metabolites, respectively (all PFOS were associated with 3 PC plasmalogens, C16:1 SM and C18:2 SM). Total PFOA, n-PFOA, and Sb-PFOA were significantly associated with 30, 23 and 34 metabolites, respectively (Supplemental Figures S4, S5; Figure 1); after FDR correction, total PFOA, n-PFOA, and Sb-PFOA were significantly associated with 14, 11, and 29 metabolites respectively (all PFOA were associated with 3 phosphatidylethanolamines and 4 triacylglycerols). Sb-PFOA p-values after FDR correction are shown in Supplemental Figure S6. Finally, PFHxS, PFNA, EtFOSAA, and MeFOSAA were all associated with 10 or fewer metabolites (Supplemental Figures S7–S10); after FDR correction, PFNA was significantly associated with C54:6 TAG, EtFOSAA was significantly associated with C18:2 SM, and PFHxS and MeFOSAA were not significantly associated with any metabolites.
Figure 1.
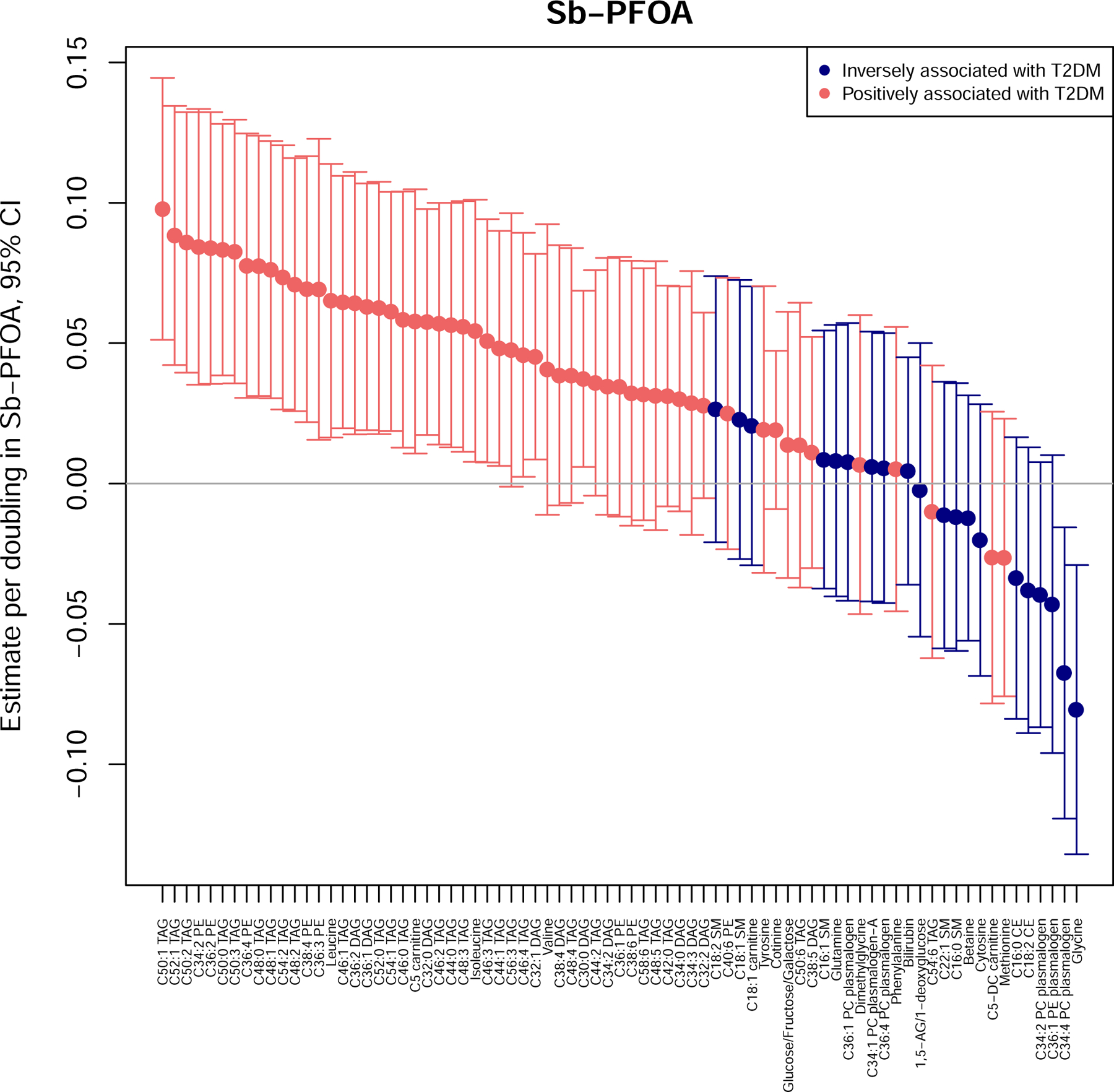
Associations of Sb-PFOA and 77 metabolites that have been previously associated with incident type 2 diabetes. Plotted estimates are from models adjusted for age, sex, race/ethnicity, use of anti-hyperlipidemic medication, use of triglyceride lowering medication, income, years of education, marital status, smoking, and family history of diabetes.
BCAAs, AAAs, glycine, glutamine
Multiple PFAS were associated with each BCAA, though few associations remained significant after FDR correction (Supplemental Table S5). Specifically, before FDR correction total PFOA, Sb-PFOA, MeFOSAA, and EtFOSAA were significantly positively associated with isoleucine; total PFOA, n-PFOA, Sb-PFOA, and PFNA were significantly positively associated with leucine; and total PFOS, n-PFOS, total PFOA, PFNA, and MeFOSAA were significantly positively associated with valine in fully adjusted models. Only Sb-PFOA and leucine remained significantly associated after FDR correction: a doubling in Sb-PFOA was associated with 0.07 (95%CI: 0.02, 0.11) SDs higher leucine.
No PFAS were associated with either of the AAAs (Supplemental Table S5). Numerous PFAS were inversely associated with glycine (total PFOA, n-PFOA, Sb-PFOA, PFNA; associations with all but PFNA remained significant after FDR correction) (Supplemental Table S5). Specifically, each doubling of total PFOA and of n-PFOA were both associated with 0.13 (95%CI: 0.04, 0.22) SDs lower glycine, and each doubling of Sb-PFOA was associated with 0.08 (95%CI: 0.03, 0.13) SDs lower glycine. N-PFOS and glutamine were positively associated, but the association was not significant after FDR correction.
The Σamino acids, ΣAAAs, and Σglycine,glutamine groups were not associated with any PFAS (Figure 2). The ΣBCAAs group was significantly associated with total PFOS, total PFOA, n-PFOA, Sb-PFOA, and EtFOSAA (Figure 2).
Figure 2.
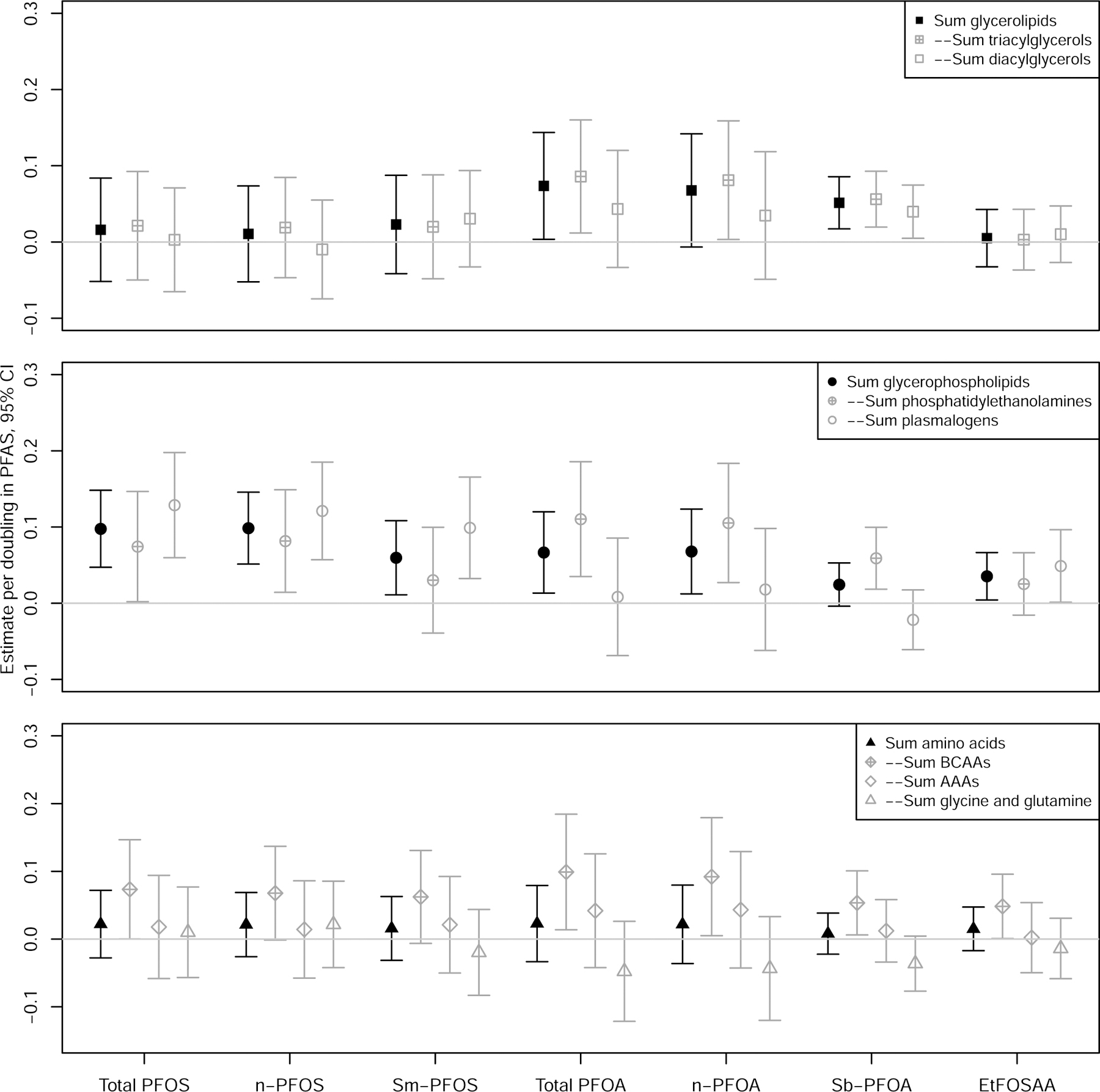
Association of each PFAS and metabolite sums in fully adjusted models. Models were adjusted for age, sex, race/ethnicity, use of anti-hyperlipidemic medication, use of triglyceride lowering medication, income, years of education, marital status, smoking, and family history of diabetes. Gray icons indicate that sums are a subset of the overall group. Abbreviations: BCAAs: branched chain amino acids; AAAs: aromatic amino acids.
Additional 70 metabolites
Of the other 70 metabolites, 47 were significantly associated with at least one PFAS; 36 metabolites remained significantly associated after FDR correction.
All sphingomyelins were positively associated with several PFAS, and the Σsphingolipids group was significantly positively associated with total PFOS, n-PFOS, Sm-PFOS, total PFOA, n-PFOA, and EtFOSAA (Supplemental Figures S1–S5, S9–S11). In individual metabolite models, each doubling in total PFOS was associated with 0.10 to 0.19 SDs higher 16:0 SM, 16:1 SM, C18:1 SM, 18:2 SM, and C22:1 SM (Supplemental Figure S1). Similar patterns of association with were seen with n-PFOS, Sm-PFOS, total PFOA, and n-PFOA (Supplemental Figures S2–S5). After FDR correction, C16:1 SM, C18:1 SM, C18:2 SM, and C22:1 SM remained significantly associated with at least one PFAS.
Similarly, the Σglycerophospholipids group was significantly positively associated with total PFOS, n-PFOS, Sm-PFOS, total PFOA, n-PFOA, and EtFOSAA (Figure 2). The Σphosphatidylethanolamines subgroup was significantly associated with total PFOS, n-PFOS, total PFOA, n-PFOA, and Sb-PFOA, while the Σplasmalogens subgroup was associated with total PFOS, Sm-PFOS, n-PFOS, and EtFOSAA (Figure 2). In individual metabolite models, after FDR correction five of eight phosphatidylethanolamines (C34:2 PE, C36:2 PE, C36:3 PE, C36:4 PE, and C38:4 PE) were associated with PFAS, including total PFOS, n-PFOS, total PFOA, n-PFOA, and Sb-PFOA (Supplemental Figures S1–S5; Figure 1). For example, each doubling in total PFOA was associated with 0.13 to 0.15 SDs higher C34:2 PE, C36:2 PE, C36:3 PE, C36:4 PE, and C38:4 PE (Supplemental Figure S4). Of six plasmalogens, four were significantly associated with at least one PFAS after FDR correction (C34:1 PC, C36:1 PC, and C36:4 PC were positively associated with total PFOS, Sm-PFOS, and n-PFOS; C34:4 PC was inversely associated with Sb-PFOA).
The Σglycerolipids group was significantly associated with total PFOA and Sb-PFOA (Figure 2); the Σdiacylglycerols subgroup was significantly associated with Sb-PFOA, while the Σtriacylglycerols subgroup was associated with total PFOA, Sb-PFOA and n-PFOA (Figure 2). Multiple individual diacylglycerols and triacylglycerols were also positively associated with total PFOA, n-PFOA, and Sb-PFOA. Interestingly, many were significantly associated with only Sb-PFOA, including C30:0 DAG, C32:0 DAG, C32:1 DAG, C44:0 TAG, C44:1 TAG, C46:0 TAG, C46:1 TAG, C46:2 TAG, C46:3 TAG, C46:4 TAG, and C48:3 TAG. Each doubling in Sb-PFOA was associated with 0.04 to 0.06 SDs higher C30:0 DAG, C32:0 DAG, C32:1 DAG, C36:1 DAG, and C36:2 DAG (Figure 1). After FDR correction, four DAGs and 18 TAGs remained significantly associated with at least one PFAS.
Sensitivity analyses
Additional adjustment for BMI and waist circumference slightly attenuated results (not shown). Analyses additionally adjusting for parity and menopausal status were restricted to women, which reduced sample size and model precision. However, inverse associations of Sb-PFOA, total PFOA, n-PFOA, and PFNA with glycine were strengthened (not shown).
When PFAS were modeled as quartiles, associations with total PFOS, total PFOA, and PFNA appeared fairly linear. On the other hand, associations of metabolites with n-PFOS, Sm-PFOS, Sb-PFOA, n-PFOA often appeared somewhat nonlinear, though confidence intervals were wide and the direction of results was consistent with linear models. For example, compared to the lowest quartile, participants in the second quartile of Sb-PFOA had 0.24 (95%CI: 0.02, 0.46) SDs lower glycine; those in the third quartile had 0.45 (95%CI: 0.22, 0.68) SDs lower glycine; and those in the fourth quartile had 0.29 (95%CI: 0.05, 0.53) SDs lower glycine (not shown).
Finally, including all PFAS (except total PFOS and total PFOA) in one model weakened associations of n-PFOS, Sm-PFOS, and n-PFOA with isoleucine, leucine, and valine, and n-PFOA with glycine. However, associations of Sb-PFOA were not substantially attenuated. For example, in multi-PFAS models, each doubling of Sb-PFOA was associated with 0.05 (95%CI: - 0.02, 0.11) SDs higher isoleucine, 0.06 (95%CI: −0.01, 0.12) SDs higher leucine and 0.07 (95%CI: 0.00, 0.14) SDs lower glycine (Supplemental Table S6). Though several PFAS are moderately to highly correlated with one another, only one condition index for the fully adjusted all-PFAS model exceeded 30 (it was 44.2); the largest variance inflation factors were 3.3–3.7 (for n-PFOS, Sm-PFOS, and n-PFOA). Taken together, these regression diagnostics suggest moderate collinearity and some variance inflation in the all-PFAS model [28]; findings from this sensitivity analysis should be interpreted with caution.
Discussion
In a population of adults at high risk of developing type 2 diabetes, plasma concentrations of PFAS were cross-sectionally associated with 36 metabolites previously shown to predict developing type 2 diabetes. Specifically, many sphingomyelins were positively associated with total PFOS, n-PFOS, total PFOA, and n-PFOA; multiple phosphatidylethanolamines were positively associated with total PFOS, n-PFOS, total PFOA, n-PFOA, and Sb-PFOA; and numerous diacylglycerols and triacylglycerols were positively associated with total PFOA, n-PFOA, and Sb-PFOA. To our knowledge, this is the first epidemiological study in adults using metabolomics profiling to explore possible mechanisms by which PFAS exposure may lead to type 2 diabetes.
We reported that certain PFAS were positively associated with the ΣBCAAs group, and with leucine specifically, in fully adjusted analyses. Numerous studies have linked higher BCAAs to risk of developing type 2 diabetes [11], though it is not clear whether BCAAs themselves impact insulin signaling or whether they just reflect obesity or metabolic dysfunction [29, 30]. In this analysis, adjustment for BMI and waist circumference attenuated but did not completely explain the positive associations of PFAS and BCAAs, suggesting that the reported associations are not due solely to confounding by obesity or central adiposity. Prior literature does not align with our findings: a mother-infant cohort [31] and a cohort of high-risk children [32] both reported null associations of PFAS with BCAAs. Associations of PFAS and BCAAs in animal models are inconsistent [33, 34]. The mechanism by which PFAS might affect BCAAs requires further study.
We also found that total PFOA, n-PFOA, and Sb-PFOA were inversely associated with glycine in fully adjusted analyses. Glycine may directly stimulate insulin release by binding receptors expressed on pancreatic islet beta cells [35], and low levels of glycine have also been linked to development of type 2 diabetes and insulin resistance [36]. To our knowledge, no epidemiologic study has reported associations of PFAS with glycine, though two animal studies found that PFAS exposure reduced glycine (among other amino acids) [33, 34]. Glycine is involved in many metabolic processes, including fatty acid oxidation and detoxification of a variety of endogenous and xenobiotic metabolites via conjugation [36]; because PFAS structurally mimic free fatty acids, PFAS exposure might upregulate fatty acid beta-oxidation, depleting glycine and other free amino acids [34].
We found that Sb-PFOA, the only PFAS predictive of incident type 2 diabetes in a previous DPP analysis [8], was significantly associated with many metabolites, and that associations with those metabolites were largely in the expected direction and strength (even after adjusting for BMI and waist circumference). Additionally, 18 of the tested metabolites, including four diacylglycerols and 11 triacylglycerols, were associated only with Sb-PFOA. Sb-PFOA is an isomer of PFOA, and during DPP recruitment (1996–1999) PFOA was widely used in consumer products. PFOA has been detected in drinking water, dairy, and seafood [10], household dust, paper food packaging, and clothing and carpet treatments [37]. The magnitude of exposure from each potential PFOA source is not well characterized: depending on assumptions about total exposure levels, exposures from contaminated food and house dust may account for close to all, or only a small fraction, of PFOA exposures [37]. In the DPP, PFOA concentrations have been positively associated with consumption of coffee and tea, and bread/rice/pasta, but were not strongly associated with meat, fish, or fats & oils consumption [38]. Due to the many possible sources of PFOA, associations of Sb-PFOA and multiple metabolites may not be completely explained by confounding by diet. The finding that Sb-PFOA is associated with many metabolites associated with type 2 diabetes risk strengthens the evidence connecting Sb-PFOA exposure and risk of type 2 diabetes.
Sb-PFOA is not as widely studied as total PFOA or other PFAS, and it is frequently detected at low concentrations or not quantified at all, though some recent research has begun to specifically quantify the effects of branched PFOA isomers. The scarcity of literature makes it challenging to contextualize our findings of strong associations for Sb-PFOA, which were robust to multiple sensitivity analyses including co-adjustment for other PFAS. However, some recent literature does support the hypothesis that Sb-PFOA is harmful. For example, two prior studies in the DPP cohort reported that baseline Sb-PFOA concentrations were associated with incident type 2 diabetes [7, 8], which aligns with our findings. In a cross-sectional study of Chinese adults, a branched PFOA isomer but not linear isomers or total PFOA was significantly positively associated with odds of hypertension [39]. Additionally, Sb-PFOA has been associated with increased uric acid and hyperuricemia; though total PFOA was also associated with these outcomes, the authors reported that the total-PFOA association with hyperuricemia appeared driven by Sb-PFOA [40]. Branched isomers of PFOS and PFOA have lower serum protein binding affinities [41] which may affect their metabolism and excretion, leading to different health effects. However, not all research supports the idea of a different metabolic effect of Sb-PFOA: in two cross-sectional studies, branched, linear, and total PFOA returned largely similar associations with metabolic syndrome and glucose homeostasis markers [42, 43]. Though some prior studies indicate a harmful role for Sb-PFOA distinct from the effects of exposure to linear isomers and total PFOA, more research is needed to determine a potential mechanism.
We reported strong positive associations of total PFOA, n-PFOA, and Sb-PFOA and glycerolipids, especially triacylglycerols. These associations were only slightly attenuated by adjustment for BMI and waist circumference. Higher triacylglycerols (triglycerides) have been associated with metabolic markers like impaired fasting glucose and incident type 2 diabetes in multiple prospective studies [44–46], including the DPP [14]. PFAS concentrations have also been associated with higher triglycerides, including in the DPP [47, 48], though not all studies found positive associations [42, 49]. We reported associations mostly with triacylglycerols having ≥50 carbons (e.g., C50:0, C50:1, C52:1, C54:2) while triacylglycerols with fewer carbons were not associated (e.g. C42:0, C44:0, C44:2, C48:5); mixed results in prior studies may reflect heterogeneity in the specific triglycerides measured in each study. Our finding that triacylglycerols were among the most common high-risk metabolites to be associated with PFAS suggests that PFAS may lead to type 2 diabetes by disrupting glycerolipid metabolism.
We found strong positive associations of total PFOS, n-PFOS, Sm-PFOS, total PFOA, n-PFOA and multiple sphingomyelins. This was unexpected, as sphingomyelins have been inversely associated with type 2 diabetes in previous studies [46, 50], including analyses in the DPP [14]. Additionally, two previous studies in children reported inverse associations of PFAS with sphingomyelins [32] and other sphingolipids [51]. Sphingomyelins are found in food, especially animal products like dairy, meat, and eggs [52]; fast food and meat have also been found to be contaminated with PFAS [53]. A recent study in the DPP examining associations of self-reported diet over the past year and PFAS concentrations reported that individuals with higher total plasma PFOS was were less likely to eat food groups low in sphingomyelins, like vegetables, fruits, and dried beans [38]. The positive association of PFOS, PFOS isomers and sphingomyelins we found may therefore be due to confounding by long-term dietary pattern.
Our results add to a small literature examining associations of PFAS and metabolomics, but few of the metabolites we tested were associated with PFAS in earlier studies. Two previous studies in adults (one in 70-year-old Swedes [54] and one in young Chinese men [55]) have tested associations of PFAS with metabolites, and reported some overlapping findings including positive associations with metabolites related to uric acid metabolism and glycerophospholipid metabolism [54, 55]. Broadly in line with these findings, we also reported positive associations of multiple PFAS with glycerophospholipids, particularly with phosphatidylethanolamines. Two studies of PFAS exposure in children reported alterations in amino acid metabolism (though altered amino acids [e.g., tyrosine, proline, and arginine] differed from the amino acids we identified as important) and lower levels of certain sphingolipids [32, 51]. Our analysis was the first to focus on metabolites associated with incident type 2 diabetes in a high-risk adult population, so some differences with other studies’ findings may be expected.
This analysis had many strengths. We leveraged a large, high-quality cohort that previously produced evidence linking PFAS and type 2 diabetes, as well as metabolomics and type 2 diabetes, to evaluate possible mechanistic pathways. We additionally tested associations of metabolites that predict type 2 diabetes years before overt disease, allowing us to explore potential mechanisms for PFAS to affect risk. Our study, which uses -omics techniques to test associations of multiple PFAS concentrations and endogenous metabolite responses, leverages a novel exposome framework to test health effects of environmental exposures [56]. Finally, after controlling for diabetes risk factors, we identified strong associations (particularly of Sb-PFOA) with multiple metabolites in the expected direction, lending our results face validity.
Our study also has limitations. First, we used a cross-sectional design, which prevented us from evaluating temporality of PFAS and metabolites and may be subject to reverse causation. However, it is unlikely that the metabolites of interest affect plasma PFAS concentrations. Second, we could not statistically test for mediation due to the cross-sectional timing of all measurements: if potential mediators (metabolites) do not occur after exposures (PFAS), tests of mediation would be meaningless. However, future longitudinal explorations of mediation could focus on testing the specific metabolites or classes highlighted here, which are promising candidates as mediators. Third, many of the tested metabolites are part of complex metabolic pathways, and their sources are incompletely understood, preventing us from completely controlling for confounding. However, we could control for major demographic and clinical confounders and conducted multiple sensitivity analyses. Fourth, we used FDR correction to adjust for multiple testing. If our analysis did not meet the assumption of positive regression dependency in subsets [27], we may have incorrectly rejected some tests. However, FDR correction is commonly used in metabolomics literature (e.g.,[14, 32, 51]) and has been shown in simulations to retain power and remain conservative when tests are not independent [57]. Finally, this study was restricted to a population at high risk of type 2 diabetes, so results may not generalize to other populations.
Conclusions
To our knowledge, this is the first study to test associations between PFAS plasma concentrations and metabolites associated with type 2 diabetes in a high-risk adult cohort. We found that plasma concentrations of certain PFAS were associated with altered concentrations of amino acids, including higher ΣBCAAs and lower glycine. We additionally reported multiple positive associations of PFOA, n-PFOA, and Sb-PFOA and triacylglycerols and diacylglycerols, as well as PFOSs, PFOAs, and glycerophospholipids. In particular, Sb-PFOA was associated with a large number of metabolites in the expected direction; this contributes to the evidence connecting Sb-PFOA concentrations and risk of incident type 2 diabetes. These results suggest that PFAS may increase risk of type 2 diabetes through dysregulation of plasma amino acids like BCAAs and glycine, glycerolipids (especially triacylglycerols), and certain glycerophospholipids (especially phosphatidylethanolamines). Further research is needed to formally test whether these pathways mediate the association of PFAS concentrations and type 2 diabetes, and further elucidate mechanisms connecting PFAS to the development of type 2 diabetes.
Supplementary Material
Plasma PFAS are associated with 36 metabolites linked to risk of type 2 diabetes.
Sb-PFOA in particular is associated with many metabolites in the expected direction.
PFAS are associated with altered amino acids, glycerolipids, & glycerophospholipids.
These metabolites are candidates to mediate associations of PFAS and type 2 diabetes.
Acknowledgements
The authors thank Andres Cardenas, Diane Gold, Pi-I Debby Lin, Russ Hauser, Tom Webster, and the metabolomics working group in the Channing Division of Network Medicine at Brigham and Women’s Hospital for constructive feedback to the analysis plan and thoughtful guidance on interpreting the results. The Research Group gratefully acknowledges the commitment and dedication of the participants of the DPP and DPPOS. Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases(NIDDK) of the National Institutes of Health (NIH) under Award Number U01 DK048489, by providing funding during DPP and DPPOS to the clinical centers and the Coordinating Center for the design and conduct of the study, and collection, management, analysis, and interpretation of the data. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, and the Department of Veterans Affairs supported data collection at many of the clinical centers. Funding was also provided by the National Institute of Child Health and Human Development, the National Institute on Aging, the National Eye Institute, the National Heart Lung and Blood Institute, the National Cancer Institute, the Office of Research on Women’s Health, the National Institute on Minority Health and Health Disparities, the Centers for Disease Control and Prevention, and the American Diabetes Association. Merck KGaA provides medication for DPPOS. DPP/DPPOS have also received donated materials from Bristol-Myers Squibb, Parke-Davis, and LifeScan Inc. LifeScan Inc., Health O Meter, Hoechst Marion Roussel, Inc., Merck-Medco Managed Care, Inc., Merck and Co., Nike Sports Marketing, Slim Fast Foods Co., and Quaker Oats Co. donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices Corp., Matthews Media Group, Inc., and the Henry M. Jackson Foundation provided support services under subcontract with the Coordinating Center. The sponsor of this study was represented on the Steering Committee and played a part in study design, how the study was done, and publication. All writing group authors had access to all data. The opinions expressed are those of the study group and do not necessarily reflect the views of the funding agencies. The Supplemental Material contains a complete list of Centers, investigators, and staff.
Role of the funding source:
The funding sources for this work had no role in the study design; collection, analysis, or interpretation of the data; writing the report; or the decision to submit the article for publication.
Funding:
This work was supported by the National Institutes of Health (T32-ES007069, K23ES024803, R01ES030101, R01ES024765).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: None.
References
- 1.Wang Z, Cousins IT, Scheringer M, Buck RC, Hungerbuhler K. Global emission inventories for C4-C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: production and emissions from quantifiable sources. Environ Int 2014;70:62–75 doi: 10.1016/j.envint.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Kotthoff M, Müller J, Jürling H, Schlummer M, Fiedler D. Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environ Sci Pollut Res 2015;22:14546–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.EPA. Basic Information on PFAS 2018. https://www.epa.gov/pfas/basic-information-pfas.
- 4.ATSDR. Per- and polyfluoroalkyl substances (PFAS) and your health. 2018. https://www.atsdr.cdc.gov/pfas/pfas-exposure.html.
- 5.Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ Sci Technol 2011;45(19):8037–45 doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- 6.Sun Q, Zong G, Valvi D, Nielsen F, Coull B, Grandjean P. Plasma concentrations of perfluoroalkyl substances and risk of type 2 diabetes: A prospective investigation among U.S. women. Environ Health Perspect 2018;126(3):037001 doi: 10.1289/EHP2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardenas A, Gold DR, Hauser R, et al. Plasma concentrations of per- and polyfluoroalkyl substances at baseline and associations with glycemic indicators and diabetes incidence among high-risk adults in the Diabetes Prevention Program Trial. Environ Health Perspect 2017;125(10):107001 doi: 10.1289/EHP1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardenas A, Hivert MF, Gold DR, et al. Associations of perfluoroalkyl and polyfluoroalkyl substances with incident diabetes and microvascular disease. Diabetes Care 2019:pii: dc182254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donat-Vargas C, Bergdahl IA, Tornevi A, et al. Perfluoroalkyl substances and risk of type II diabetes: a prospective nested case-control study. Environment International 2019;123:390–98. [DOI] [PubMed] [Google Scholar]
- 10.Mancini FR, Rajaobelina K, Praud D, et al. Nonlinear associations between dietary exposures to perfluorooctanoic acid (PFOA) or perfluorooctane sulfonate (PFOS) and type 2 diabetes risk in women: findings from the E3N cohort study. Int J Hygiene Environ Health 2018;221(7):1054–60. [DOI] [PubMed] [Google Scholar]
- 11.Guasch-Ferre M, Hruby A, Toledo E, et al. Metabolomics in prediabetes and diabetes: A systematic review and meta-analysis. Diabetes Care 2016;39:833–46 doi: 10.2337/dc15-2251/-/DC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gängler S, Waldenberger M, Artati A, et al. Exposure to disinfection byproducts and risk of type 2 diabetes: a nested case–control study in the HUNT and Lifelines cohorts. Metabolomics 2019;15(60). [DOI] [PubMed] [Google Scholar]
- 13.Walford GA, Ma Y, Clish C, Florez JC, Wang TJ, Gerszten RE. Metabolite profiles of diabetes incidence and intervention response in the Diabetes Prevention Program. Diabetes 2016;65:1424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen ZZ, Liu J, Morningstar J, et al. Metabolite profiles of incident diabetes and heterogeneity of treatment effect in the Diabetes Prevention Program. Diabetes 2019;68(12):2337–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenmai AK, Taxvig C, Svingen T, et al. Fluorinated alkyl substances and technical mixtures used in food paper-packaging exhibit endocrine-related activity in vitro. Andrology 2016;4(4):662–72 doi: 10.1111/andr.12190. [DOI] [PubMed] [Google Scholar]
- 16.Wolf CJ, Schmid JE, Lau C, Abbott BD. Activation of mouse and human peroxisome proliferator-activated receptor-alpha (PPARalpha) by perfluoroalkyl acids (PFAAs): further investigation of C4-C12 compounds. Reprod Toxicol 2012;33(4):546–51 doi: 10.1016/j.reprotox.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Hu W, Jones PD, Celius T, Giesy JP. Identification of genes responsive to PFOS using gene expression profiling. Environ Toxicol Pharmacol 2005;19(1):57–70. [DOI] [PubMed] [Google Scholar]
- 18.Yu N, Wei S, Li M, et al. Effects of perfluorooctanoic acid on metabolic profiles in brain and liver of mouse revealed by a high-throughput targeted metabolomics approach. Scientific Reports 2016;6:23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: baseline characteristics of the randomized cohort. Diabetes Care 2000;23(11):1619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato K, Basden BJ, Needham LL, Calafat AM. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A 2011;1218:2133–37. [DOI] [PubMed] [Google Scholar]
- 21.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 1990;5(1):46–51. [Google Scholar]
- 22.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology 2004;15(5):615–25. [DOI] [PubMed] [Google Scholar]
- 23.Sagiv SK, Rifas-Shiman SL, Webster TF, et al. Sociodemographic and perinatal predictors of early pregnancy per- and polyfluoroalkyl substance (PFAS) concentrations. Environ Sci Technol 2015;49(19):11849–58 doi: 10.1021/acs.est.5b02489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLaughlin T, Reaven G, Abbasi F, et al. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol 2005;96(3):399–404. [DOI] [PubMed] [Google Scholar]
- 25.Petrakis D, Vassilopoulou L, Mamoulakis C, et al. Endocrine disrupotrs leading to obesity and related dieases. Int J Environ Res Public Health 2017;14(10):1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 27.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Statistics 2001;29(4):1165–88. [Google Scholar]
- 28.Dormann CF, Elith J, Bacher S, et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013;36(1):27–46. [Google Scholar]
- 29.Tobias DK, Clish C, Mora S, et al. Dietary Intakes and Circulating Concentrations of Branched-Chain amino acids in relation to incident type 2 diabetes risk among high-risk women with a history of gestational diabetes mellitus. Clin Chem 2018;64(8):1203–10 doi: 10.1373/clinchem.2017.285841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nature Reviews Endocrinology 2014;10(12):723–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGlinchy A, Sinioja T, Lamichhane S, et al. Prenatal exposure to environmental chemicals modulates serum phospholipids in newborn infants, increasing later risk of type 1 diabetes. bioxrv 2019. doi: 10.1101/588350. [DOI] [Google Scholar]
- 32.Alderete TL, Jin R, Walker DI, et al. Perfluoroalkyl substances, metabolomic profiling, and alterations in glucose homeostasis among overweight and obese Hispanic children: A proof-of-concept analysis. Environment International 2019;126:445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Ding L, Fang X, et al. Biological responses to perfluorododecanoic acid exposure in rat kidneys as determined by integrated proteomic and metabonomic studies. PLoS One 2011;6(6):e20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kariuki MN, Nagato EG, Lankadurai BP, Simpson AJ, Simpson MJ. Analysis of sub-lethal toxicity of perfluorooctane sulfonate (PFOS) to Daphnia magna using 1H nuclear magnetic resonance-based metabolomics. Metabolites 2017;7(2):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan-Do R, Duong E, Manning Fox JE, et al. A glycine-insulin autocrine feedback loop enhances insulin secretion from human β-cells and is impaired in type 2 diabetes. Diabetes 2016;65(8):2311–21. [DOI] [PubMed] [Google Scholar]
- 36.Alves A, Bassot A, Bulteau AL, Priola L, Morio B. Glycine metabolism and its alteration in obesity and metabolic diseases. Nutrients 2019;11:1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbuhler K. Estimating consumer exposure to PFOS and PFOA. Risk Analysis 2008;28(2):251–69. [DOI] [PubMed] [Google Scholar]
- 38.Lin PD, Cardenas A, Hauser R, et al. Dietary characteristics associated with plasma concentrations of per- and polyfluoroalkyl substances among adults with pre-diabetes: Cross-sectional results from the Diabetes Prevention Program Trial. Environment International 2020;137(105217). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bao W-W, Qian Z, Geiger SD, et al. Gender-specific associations brween serum isomers of perfluoroalkyl substances and blood pressure among Chinese: isomers of C8 Health Project in China. Sci Total Environ 2017;607–608:1304–12. [DOI] [PubMed] [Google Scholar]
- 40.Zeng XW, Lodge CJ, Dharmage SC, et al. Isomers of per- and ployfluoroalky substances and uric acid in adults: isomers of C8 Health Project in China. Environ Int 2019;133(Pt A):105160. [DOI] [PubMed] [Google Scholar]
- 41.Beesoon S, Genuis SJ, Benskin JP, Martin JW. Exceptionally high serum concentrations of perfluorohexanesulfonate in a Canadian family are linked to home carpet treatment applications. Environ Sci Technol 2012;46(23):12960–7 doi: 10.1021/es3034654. [DOI] [PubMed] [Google Scholar]
- 42.Liu HS, Wen LL, Chu PL, Lin CY. Association among total serum isomers of perfluorinated chemicals, glucose homeostasis, lipid profiles, serum protein, and metabolic syndrome in adults: NHANES, 2013–2014. Environ Pollut 2018;232:73–79. [DOI] [PubMed] [Google Scholar]
- 43.Tian YP, Zeng XW, Bloom MS, et al. Isomers of perfluoroalkyl substances and overweight status among Chinese by sex status: isomers of C8 Health Project in China. Environ Int 2019;124:130–38. [DOI] [PubMed] [Google Scholar]
- 44.Beshara A, Cohen E, Goldberg E, Lilos P, Garty M, Krause I. Triglyceride levels and risk of type 2 diabetes mellitus: A longitudinal large study. J Investig Med 2016;64(2):383–87. [DOI] [PubMed] [Google Scholar]
- 45.Lin SX, Berlin I, Younge R, et al. Does elevated plasma triglyceride level independently predict impaired fasting glucose?: The multi-ethnic study of atherosclerosis (MESA). Diabetes Care 2013;36(2):342–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Razquin C, Toledo E, Clish CB, et al. Plasma lipidomic profiling and risk of type 2 diabetes in the PREDIMED Trial. Diabetes Care 2018;41(12):2617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin PD, Cardenas A, Hauser R, et al. Per- and polyfluoroalkyl substances and blood lipid levels in pre-diabetic adults—longitudinal analysis of the diabetes prevention program outcomes study. Environment International 2019;129:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steenland K, Tinker S, Frisbee S, Ducatman A, Vaccarino V. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am J Epidemiol 2009;170(10):1268–78. [DOI] [PubMed] [Google Scholar]
- 49.Fisher M, Arbuckle TE, Wade M, Haines DA. Do perfluoroalkyl substances affect metabolic function and plasma lipids?--Analysis of the 2007–2009, Canadian Health Measures Survey (CHMS) Cycle 1. Environ Res 2013;121:95–103 doi: 10.1016/j.envres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Kikas P, Chalikias G, Tziakas D. Cardiovascular implications of sphingomyelin presence in biological membranes. Eur Cardiol 2018;13(1):42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kingsley SL, Walker DI, Calafat AM, et al. Metabolomics of childhood exposure to perfluoroalkyl substances: a cross‑sectional study. Metabolomics 2019;15(95) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Norris GH, Blesso CN. Dietary and endogenous sphingolipid metabolism in chronic inflammation. Nutrients 2017;9(1180). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tittlemeier SA, Pepper K, Seymour C, et al. Dietary exposure of Canadians to perfluorinated carboxylates and perfluorooctane sulfonate via consumption of meat, fish, fast foods, and food items prepared in their packaging. Journal of Agricultural and Food Chemistry 2007;55:3203–10. [DOI] [PubMed] [Google Scholar]
- 54.Salihovic S, Fall T, Ganna A, et al. Identification of metabolic profiles associated with human exposure to perfluoroalkyl substances. Journal of Exposure Science & Environmental Epidemiology 2018. [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Liu L, Zhang W, et al. Serum metabolome biomarkers associate low-level environmental perfluorinated compound exposure with oxidative /nitrosative stress in humans. Environmental Pollution 2017;229:168–76. [DOI] [PubMed] [Google Scholar]
- 56.Haddad N, Andrianou XD, Makris KC. A scoping review on the characteristics of human exposome studies. Current Pollution Reports 2019;5:378–93. [Google Scholar]
- 57.Kim KI, van de Wiel MA. Effects of dependence in high-dimensional multiple testing problems. BMC Bioinformatics 2008;9(114). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


