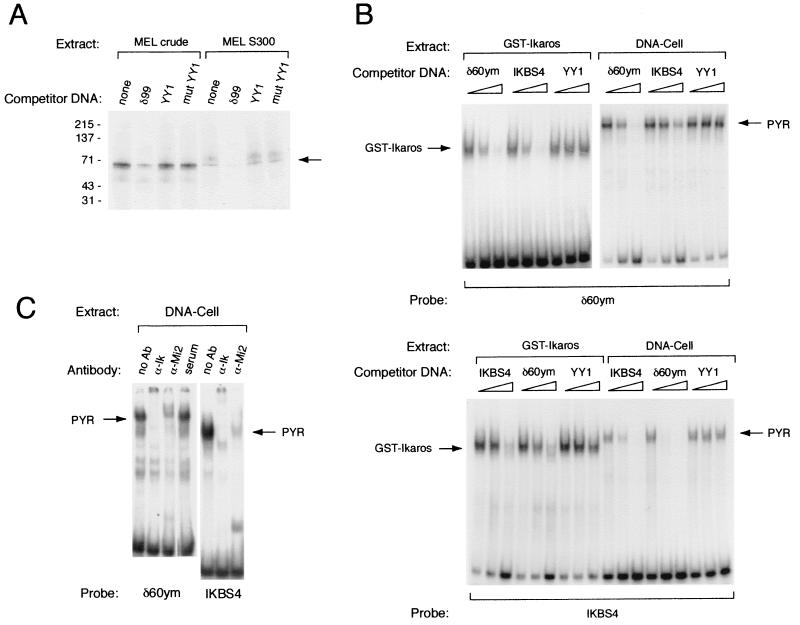
FIG. 3.
Ikaros functions as the DNA-binding subunit of the PYR complex. (A) PYR complex has a 60- to 70-kDa DNA-binding subunit as detected by photoactivated cross-linking using both MEL crude nuclear extract and Sephacryl S300 chromatography fractions containing peak PYR complex DNA-binding activity. The band corresponding to this activity is competed away with excess unlabeled δ99 DNA but not by consensus (YY1) or mutant (mut YY1) YY1-binding-site DNA. (B) The PYR complex and GST-Ikaros have similar DNA-binding specificities. Upper panel: Gel shift assay using labeled δ60ym DNA probe, GST-Ikaros protein (left lanes), and partially purified PYR complex (DNA-cellulose column peak fractions [DNA-Cell], right lanes). Both GST-Ikaros and the PYR complex bind δ60ym, and these binding activities are competed away with unlabeled δ60ym DNA and DNA from a previously described high-affinity Ikaros-binding site, IKBS4, but not with YY1-binding- site DNA. The various competitor DNAs were used at 0-, 10-, and 100-fold molar excesses. Lower panel: Gel shift assay using labeled IKBS4 DNA probe, GST-Ikaros protein (left), and DNA cellulose-purified PYR complex (right). Both GST-Ikaros and the PYR complex bind labeled IKBS4, and these binding activities are competed away with unlabeled IKBS4 and δ60ym, but not with YY1-binding-site, DNA. (C) Antibodies to Ikaros and Mi-2 supershift the activity in DNA-cellulose-purified fractions that binds δ60ym (left lanes) and IKBS4 (right lanes).