FIG. 6.
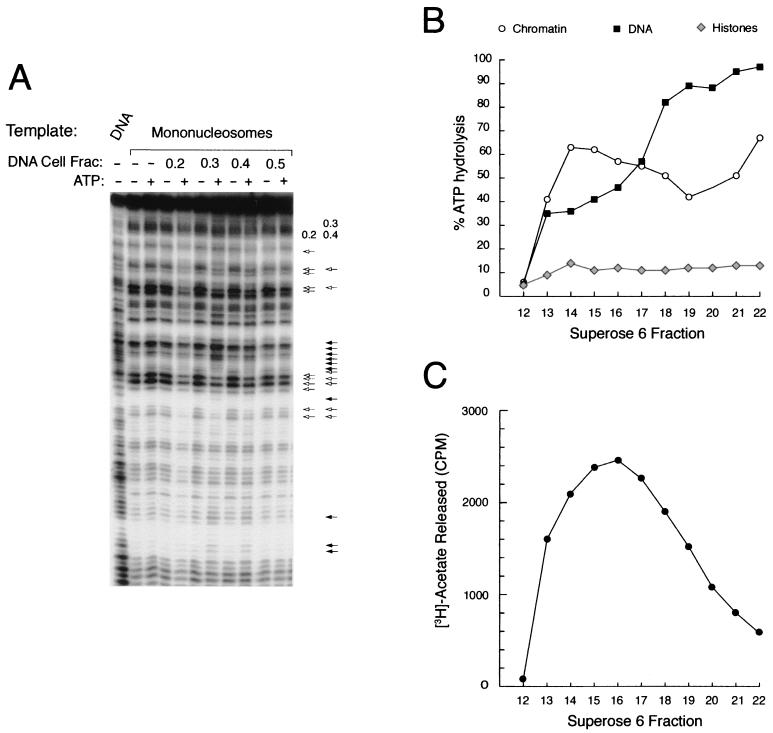
The PYR complex is associated with nucleosome remodeling and histone deacetylase activities. (A) Calf thymus DNA cellulose column fractions with peak PYR complex DNA-binding activity remodel mononucleosomes in an ATP-dependent manner. DNA cellulose column fractions (Fig. 4C) were tested for the ability to remodel a radiolabeled probe (IFNβ-110) packaged into a mononucleosome template in the presence (+) or absence (−) of ATP. Remodeling activity, indicated by the combination of increased (filled arrows) and decreased (open arrows) DNase I sensitivity in the presence of ATP, is found in the 0.3 and 0.4 M NaCl fractions. A different pattern of activity seen in the 0.2 M fraction, suggestive of DNase I protection, is also indicated. (B) Superose 6-purified PYR complex is associated with DNA-dependent ATPase activity. DNA-dependent ATPase activity, as measured by percent ATP hydrolysis, is detected in all of the fractions off the Superose 6 column (Fig. 4D) except fraction 12. Two different activities elute off the column, a high-molecular-weight activity that prefers chromatin as its substrate (fractions 13 through 17) and a lower-molecular-weight activity that prefers naked DNA (fractions 18 through 22). No appreciable ATPase activity is seen in control reactions that use purified histones as the substrate. (C) Superose 6-purified PYR complex is associated with histone deacetylase activity. Histone deacetylase activity, as measured by counts per minute of released [3H]acetate, is detected in the high-molecular-weight fractions (peak activity in fractions 13 through 18).