FIG. 3.
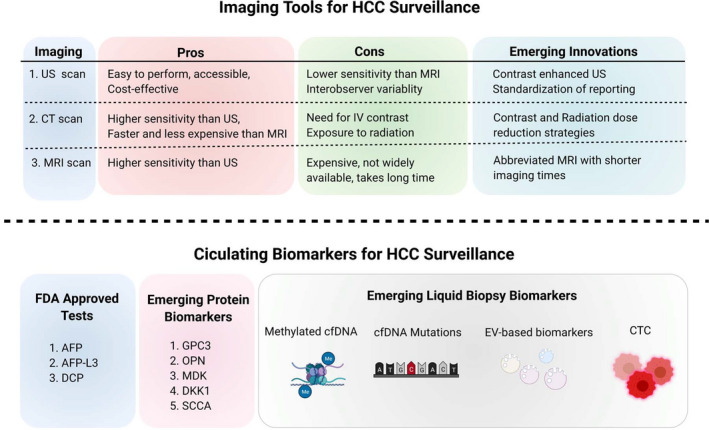
Existing and emerging biomarkers for HCC surveillance. US, CT, and MRI imaging modalities all have their pros and cons when used in the surveillance of at‐risk patients. Emerging innovations in these modalities, mostly by way of adjustments to imaging protocols and addition of agents that allow for more accurate detection, will improve upon existing limitations. AFP, AFP‐L3, and DCP are known FDA‐approved circulating biomarkers for HCC surveillance, but several emerging protein biomarkers (GPC3, OPN, MDK, DKK1, and SCCA) are proving to be clinically promising. The newest wave of innovation in tools for HCC surveillance lies in liquid biopsy biomarkers, which include methylated cfDNA, cfDNA mutations, EV‐based biomarkers, and CTC detection. Abbreviations: DKK1, Dickkopf‐1; IV, intravenous; MDK, midkine; OPN, osteopontin; SCCA, squamous cell carcinoma antigen.
