Figure 1: Study selection flow chart:
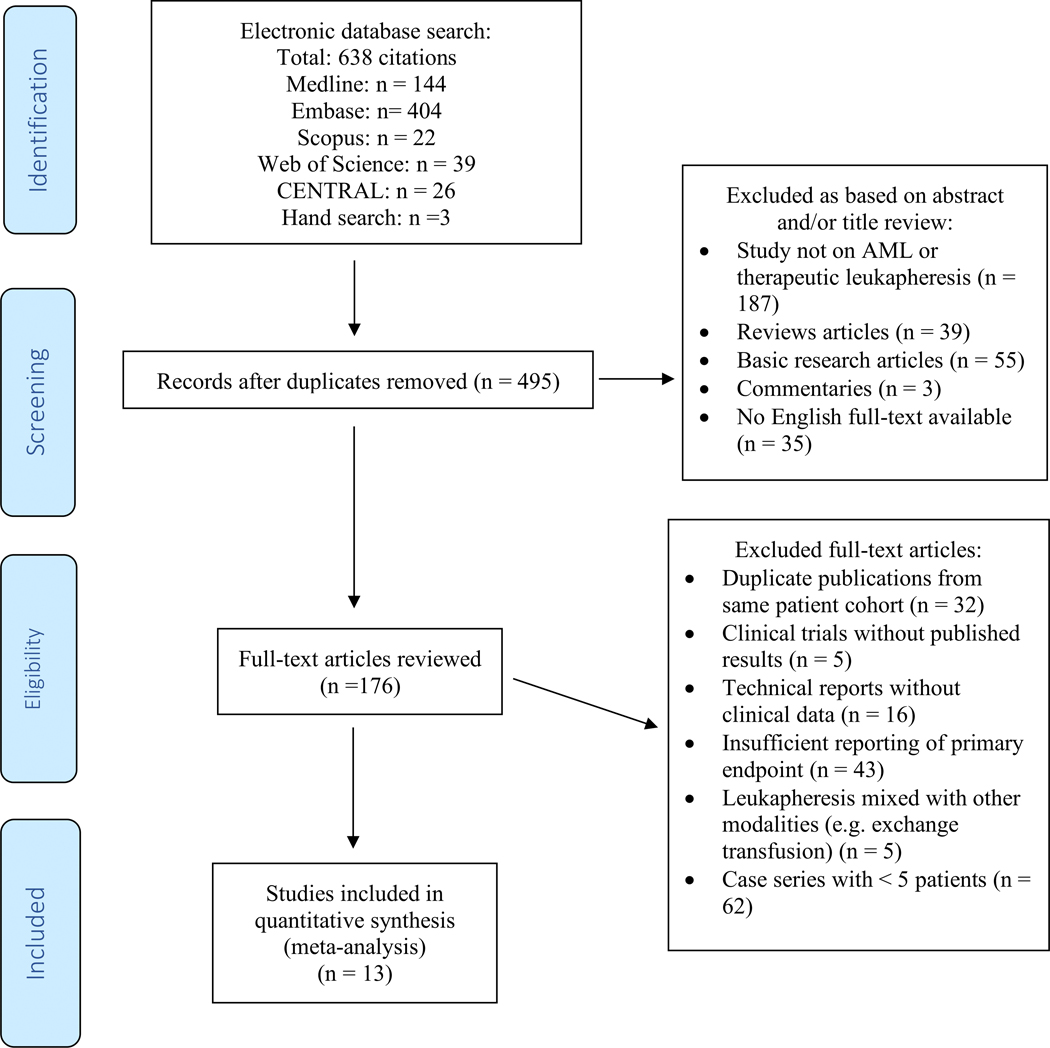
MEDLINE and EMBASE via Ovid, the COCHRANE registry of clinical trials (CENTRAL), Scopus and the Web of Science electronic databases were searched using a combination a combination of the following free-text terms linked by Boolean operators: [Acute myeloid leukemia OR AML] AND [Leukapheresis OR Leukocytapheresis]. We also conducted a manual search of the reference lists of included studies. After removal of duplicates, the titles and abstracts were reviewed and based on predefined criteria studies were excluded during this stage if they were (I) not on AML or therapeutic leukapheresis, (II) review articles, (III) basic research articles, (IV), commentaries without reporting of original data, or (V) if an English full-text was unavailable. Subsequently, full texts of the remaining studies were reviewed for eligibility and studies were excluded if they (I) were duplicate publications from same patient cohort, (II) were clinical trials without published results, (III) were technical reports without clinical data, (IV) had insufficient reporting of primary endpoint (e.g. single-arm studies), (V) only reported results of leukapheresis combined with other modalities (e.g. exchange transfusion), or (VI) were case series with < 5 patients. The final cohort for inclusion in this meta-analysis included 13 studies.
