Abstract
Background:
Few studies have examined longitudinal asthma incidence rates from a public health surveillance perspective.
Objective:
Calculate descriptive asthma incidence rates in children over time considering demographics and parental asthma history.
Methods:
Data from nine US birth cohorts were pooled into one population covering 1980-2017. The outcome was earliest parental report of a doctor diagnosis of asthma. Incidence rates per 1,000 person-years were calculated.
Results:
The 6,283 children were 55% European-American (EA), 25.5% African-American (AA), 9.5% Mexican-Hispanic American (MA) and 8.5% Caribbean-Hispanic American (CA). Average follow-up was 10.4 years (SD=8.5 years, median=8.4) totaling 65,291 person-years, with 1789 asthma diagnoses yielding a crude incidence rate of 27.5 per 1000 person-years (95% CI 26.3-28.8). Age-specific rates were highest among children 0-4 years, notably from 1995-1999, with a decline in EA/MAs in 2000-2004 followed by a decline in AA/CAs in 2010-2014. Parental asthma history was associated with statistically significantly increased rates. Incidence rates were similar and higher in AA and CA compared to lower but similar rates in EA and MA. Differential rates by sex from birth through adolescence principally resulted from a decline in male but relatively stable female rates.
Conclusions:
US childhood asthma incidence rates varied dramatically by age, sex, parental asthma history, race and calendar year. Higher rates in the 0-4 year-olds, particularly in AA/CA males with a parental history, and changes in rates over time and by demographic factors, suggests that asthma is driven by complex interactions between genetic susceptibility and variation in time-dependent environmental and social factors.
Keywords: epidemiology, incidence rates, pediatric asthma, sex, family history, time, United States
Capsule Summary
Childhood asthma incidence rates varied by decade of birth, age, race/ethnicity, child sex, region and parental history. These changes, most notable in children 0-4 years old, suggest strong environmental and societal influences on genetic asthma propensity.
Introduction
An incidence rate is a fundamental epidemiological measure, which, unlike prevalence, is independent of disease duration and remission.1 As such, incidence rates are the most valuable data for generating hypotheses related to disease etiology.2 In the United States, there are legal requirements to report initial diagnoses of most infectious diseases and cancers to public health authorities, which provide national surveillance systems and detailed descriptive data for these conditions. Routine reporting of incidence rates by associated demographic variables from these surveillance systems over time have been the foundation of numerous hypotheses and discoveries.3 However, there is remarkably little standardized information regarding incidence rates for either childhood or adult asthma, in the United States or worldwide.4-9 Although some have proposed that asthma become a reportable disease,10, 11 this idea has never been promulgated and there are no long term established U.S. population-based registries identifying new cases, in contrast, for example, to the National Cancer Institute’s cancer surveillance system that has existed since the early 1970s.12, 13 A careful search of the literature reveals few studies reporting incidence rates for pediatric asthma.14-23 Most are calculated using data collected for other purposes and cover limited calendar time-periods, age groups, geographic areas and/or racial/ethnic groups.24-28 These incidence rates are frequently based on a number of suppositions such as assuming the timing of diagnoses to be midpoint between two questionnaires occurring over a prolonged time interval, or on the use of questionnaires not specifically designed, standardized and validated to collect information on the occurrence of asthma. More studies report cumulative incidence measures,29-32 a measure of the proportion of a population that has developed the disease, which is not a rate but a ratio, and a measure of risk.2
Several epidemiological dogmas exist for pediatric asthma, based largely on prevalence data. For example, it is inferred from prevalence data33, 34 that Puerto Rican children develop more asthma, followed by Americans of African origins, Americans of European geographic ancestry and least frequently, Mexican-Americans.35, 36 Reviews of the scientific literature suggest that asthma prevalence is more common in boys than girls until around puberty when the pattern reverses, presumably because of an increase in asthma incidence rates among girls.37 Other research has attempted to evaluate whether the incidence rates of asthma are changing for children born in more recent decades, which suggests a need to investigate changes in the environment occurring during the relevant time-periods. Reports propose that, in the U.S. and other developed countries, an increase in asthma occurred in the 1980s and 1990s and has since plateaued or even decreased, but most of the data cited to justify these observations come from health care utilization information or prevalence measures.38-42
To address this knowledge gap, we have calculated asthma incidence rates using pooled birth cohort data from the Children’s Respiratory and Environment Workgroup (CREW), funded through the NIH Environmental influences on Child Health Outcomes (ECHO) program (www.echochildren.org). CREW brings together a dozen demographically diverse asthma-focused birth cohorts from across the US in an effort to harmonize and subsequently combine data across these study populations.43 This collaboration allowed a retrospective construction of a “surveillance system” for these geographically defined populations of newborns who have been specifically followed for the development of asthma. This approach is complementary to another analysis of asthma incidence rates in 31 ECHO cohorts that includes some CREW cohorts,14 in which cohort-specific incidence rates were combined in a meta-analysis, as opposed to pooling individual data. The sample size was larger in the ECHO-wide study; however, the methodological approach did not permit multi-variable modeling or include calendar year of diagnosis (basic to surveillance) or detailed racial/ethnic categories. Further, in CREW, all the cohorts were focused on asthma and thus included more frequent survey questions regarding asthma , thereby increasing confidence in the date of asthma diagnosis.
Our goal was to use the harmonized and subsequently pooled data across the CREW cohorts to directly calculate overall and subgroup-specific asthma incidence rates from 1980 through 2017, considering the fundamental epidemiological variables of “time, place and person”.2 An advantage relative to a typical surveillance system is that in the CREW studies the parental history of asthma was recorded and used as a surrogate for potential genetic susceptibility.44
Methods
We used data from nine of the 12 longitudinal US birth cohorts that are part of the CREW consortium within the ECHO program, described in detail by Gern et al.43 to examine asthma incidence rates. Three of the CREW cohorts were not included because the youngest children were enrolled in relatively recent years (as late as 2014, 2016 and 2019, respectively) and could only contribute minimal person-years of follow-up per child at the time the data were harmonized and pooled across cohorts for these analyses. A data sharing protocol and a data use agreement were approved by the local institutional review boards for each participating cohort. Each cohort provided data to a single biostatistician (SLH) who, with the lead epidemiologist (CCJ), worked through a back and forth process among the individual cohort data managers and investigators to address initial differences in the timing and wording of data collection tools and variations in data management, both before and after the data were eventually pooled for harmonization. The follow-up data provided were up to date from each cohort as of 12-31-2017.
Demographic Variables and Definition of Outcome
Children included in the rates were born from 1980 through 2007 and followed through the end of 2017, a date of asthma diagnosis, or until dropped from their respective birth cohort due to loss of follow-up, death or withdrawal. Geography was classified into three US regions based on cohort location: Northeast (Baltimore MD, Boston MA, New York City NY), Midwest (Detroit MI, Cincinnati OH, Madison WI and St. Louis, MO) and Southwest (Tucson, AZ). Date of birth and sex at birth were available for all children. Race/ethnicity was classified into five self-identified categories: European-American (European or EA), African-American (African or AA), Caribbean-American Hispanic (Caribbean or CA), Mexican-American Hispanic (Mexican or MA) and other. Children were categorized into these groups based on their survey data and in a few instances by further checks with a cohort’s research staff regarding available genetic ancestry data or other information helpful for this classification. Parental history of disease was considered positive if either birth parent was reported to have a history of a doctor diagnosis of asthma.
An incident case of asthma was defined as the first occurrence of an asthma diagnosis by a doctor based the date of diagnosis as reported by a parent, usually the mother. If the exact date of diagnosis was not recalled, it was assumed to be at the median point between the date of the questionnaire where the diagnosis was reported and the previous questionnaire. Our objective was to retrospectively construct population data and incident cases as it would have been collected by a public health surveillance system that accepts results from physicians and/or associated medical laboratories without future modifications; therefore, we did not place any restrictions on these first reports.
Statistical Analyses
Incidence rates per 1000 person-years and 95% confidence intervals (CI) derived from nine CREW cohorts were computed by cohort and across the combined cohorts by calendar time, decade of birth, region, age groups (0-4, 5-9, 10-14, 15-19 and 20-35 years), race/ethnicity, child sex, and parental history of asthma. Rates were first calculated in the standard way that is often referred to as a “crude” incidence rate and is defined by the number of new asthma cases divided by the summed person-years at risk during a defined observation time. Censored subjects were attributed to be disease-free for half of the lost time, for example a person followed for one year but lost to follow-up at year two contributes an additional half-year of disease-free time to the denominator. Rates were then calculated for each sub-category of interest (e.g. calendar time (year categories), decade of birth, geographic region, age, race/ethnicity, sex and parental history of asthma). “Decade Born” can suggest hypotheses related to environmental changes that may have affected all age groups over the time-period followed. Stratified tables are presented for ease of interpretation. Multivariable Poisson regression models using the robust Huber-White sandwich variance estimator were performed to calculate adjusted incidence rate ratios (aIRRs) and 95% CIs.45, 46 All adjusted models included the following as potential confounders: decade of birth, region, age, race/ethnicity, sex, and parental history of asthma. Parental history of asthma, child sex, race/ethnicity, age, region, and decade of birth were analyzed as potential effect modifiers.
Results
Table 1 shows the characteristics of the children included in the analyses and crude incidence rates (for information by individual cohort see Supplement Table 1 and Figure 1). The 37-year time-period of observation for asthma diagnosis ranged from 1980 to 2017 for the 6283 children, with the most person-years of observation between 2000 and 2009. The average person-time contributing to the sample for these analyses, starting at birth (birth years ranged from 1980-2007), was 10.4 years (sd=8.5; median=8.4) for a total of 65,291 person-years. The oldest cohort participants had reached 34.8 years and the youngest were 12.7 years at last date of follow up in 2017.
Table 1.
Overall univariate crude doctor-diagnosed asthma incidence rates (IR) per 1000 person-years, by epidemiological characteristics*
| Characteristic | Level | N (%) | Person- years |
Number of cases of asthma |
IR (95% CI) Per 1000 |
|---|---|---|---|---|---|
| All subjects | - | 6283 | 65291 | 1798 | 27.5 (26.3, 28.8) |
| Calendar Time of Surveillance ** | 1980-1984 | 1225 (19.5%) | 2860 | 19 | 6.6 (4.0, 10.4) |
| 1985-1989 | 1884 (30.0%) | 6123 | 123 | 20.1 (16.7, 24.0) | |
| 1990-1994 | 1777 (28.3%) | 7690 | 178 | 23.1 (19.9, 26.8) | |
| 1995-1999 | 2494 (39.7%) | 8609 | 262 | 30.4 (26.9, 34.3) | |
| 2000-2004 | 3813 (60.7%) | 13056 | 357 | 27.3 (24.6, 30.3) | |
| 2005-2009 | 4628 (73.7%) | 16286 | 683 | 41.9 (38.9, 45.2) | |
| 2010-2014 | 2507 (39.9%) | 8761 | 152 | 17.3 (14.7, 20.3) | |
| 2015-2017 | 1204 (19.2%) | 1904 | 24 | 12.6 (8.1, 18.8) | |
| Decade Born | 1980-1989 | 2058 (32.6) | 33731 | 562 | 16.7 (15.3, 18.1) |
| 1990-1999 | 1098 (17.5) | 11169 | 390 | 34.9 (31.5, 38.6) | |
| 2000-2009 | 3127 (49.8) | 20391 | 846 | 41.5 (38.7, 44.4) | |
| Geographical Region | Northeast | 1535 (24.4%) | 13646 | 622 | 45.6 (42.1, 49.3) |
| Midwest | 3044 (48.5%) | 25404 | 706 | 27.8 (25.8, 29.9) | |
| Southwest | 1704 (27.1%) | 26240 | 470 | 17.9 (16.3, 19.6) | |
| Child Age during Follow-up** (yrs) | 0-4 | 6283 (100%) | 25520 | 1011 | 39.6 (37.2, 42.1) |
| 5-9 | 4365 (69.5%) | 17549 | 440 | 25.1 (22.8, 27.5) | |
| 10-14 | 2815 (44.8%) | 10471 | 188 | 18.0 (15.5, 20.7) | |
| 15-19 | 1649 (26.2%) | 5950 | 77 | 12.9 (10.2, 16.2) | |
| 20-35 | 712 (11.3%) | 5800 | 82 | 14.1 (11.2, 17.5) | |
| Child Race/Ethnicity | European American | 3268 (55.0) | 40184 | 829 | 20.6 (19.2, 22.1) |
| African American | 1515 (25.5) | 9813 | 504 | 51.4 (47.0, 56.0) | |
| Mexican American | 563 (9.5) | 7338 | 156 | 21.3 (18.1, 24.9) | |
| Caribbean American | 505 (8.5) | 3789 | 196 | 51.7 (44.7, 59.5) | |
| Other | 92 (1.5%) | 955 | 17 | 17.8 (10.4, 28.5) | |
| Child Sex | Male | 3172 (50.5) | 30901 | 984 | 31.8 (29.9, 33.9) |
| Female | 3110 (49.5) | 34389 | 814 | 23.7 (22.1, 25.4) | |
| Parental History of Asthma | None | 3401 (64.5) | 41344 | 725 | 17.5 (16.3 , 18.9) |
| Paternal only | 645 (12.2%) | 6308 | 239 | 37.9 (33.2 , 43.0) | |
| Maternal only | 1028 (19.5%) | 8342 | 422 | 50.6 (45.9 , 55.7) | |
| Both parents | 198 (3.8%) | 1248 | 106 | 84.9 (69.5, 102.7) | |
| Either or both parents | 2017 (37.2) | 16927 | 831 | 49.1 (45.8, 52.5) |
Child sex missing for one child, race/ethnicity missing for 432 children, parental history of asthma missing for 1,011 children.
the population numbers in these categories are not mutually exclusive; children in the cohorts are followed as they age over calendar time from the point of enrollment (birth date) until they are censored due to cessation of follow up or asthma diagnosis.
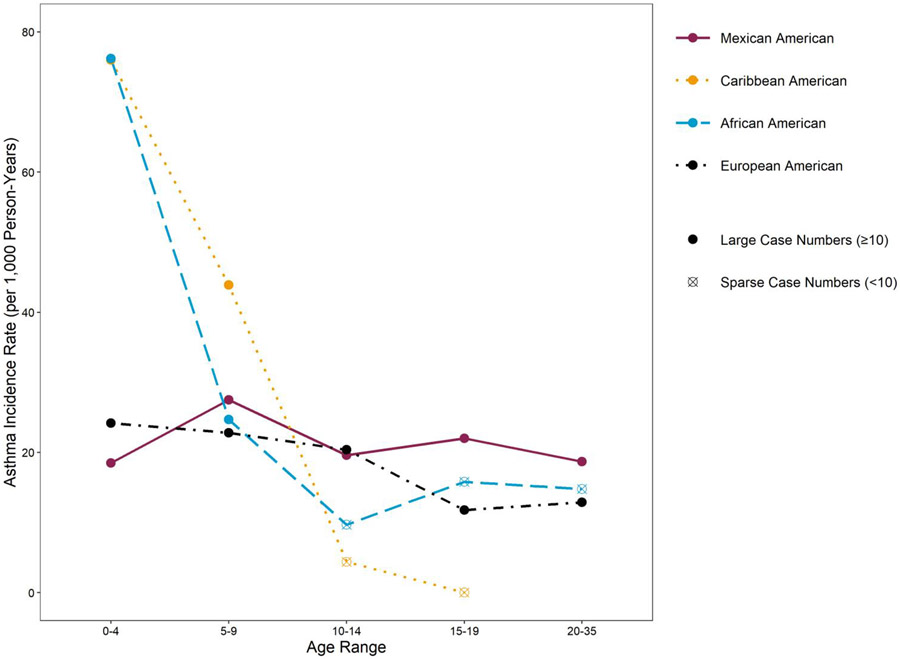
Figure 1.
Doctor-diagnosed asthma incidence rates per 1000 person-years, by age and four individual race/ethnicity categories; n of population = 5035.
In the combined cohorts at time of birth, 55% of the children were of European descent (European-Americans), 25.5% African-American, 9.5% Mexican-American Hispanic, and 8.5% Puerto Rican or Dominican (Caribbean-American Hispanic). Other race/ethnic groups comprised only 1.5% of the population and were excluded from further analyses. Four of the nine cohorts required a parental allergic history (asthma, allergy or sensitization) with varying definitions for eligibility (see Description of Cohorts in Supplement). Using the pooled data, a parental history of asthma (either mother, father or both) was reported by 37.2% of the parents across all the cohorts.
There were 1798 cases of physician-diagnosed asthma with an overall crude incidence rate (IR) of 27.5 per 1000 person-years; 95% confidence interval (CI) 26.3-28.8 (Table 1). Considering calendar period of diagnoses, rates were highest from 1995-2009, with all year categories having an IR of >25 per 1,000 person-years during this time-period and a peak at 41.9 in 2005-2009 (CI 38.9-45.2). However, while the proportions by child sex remained constant over calendar time in the cohorts, the cohorts born earliest came from general population sampling frames (rather than being required to have a family history of asthma or allergies) and were predominantly of European ethnicity ( Supplement Figure 2), both variables associated with lower IRs (Table 1, Supplement Tables 2 and 3). Therefore, subsequent analyses by calendar time and decade of birth stratified the data by race/ethnicity and parental history.
Interestingly, the IRs were nearly identical, and more than doubled for African-Americans and Caribbean-Americans (51.4 per 1000 person-years; 95% CI 47.0-56.0 and 51.7 per 1000 person-years; 95% CI 44.7-59.5 per 1000 person-years, respectively) as opposed to nearly identical rates in European-Americans (20.6 per 1000 person-years; 95% CI 19.2-22.1) and Mexican-Americans (21.3 per 1000 person-years; 95% CI 18.1-24.9) (Table 1). When stratified by parental history and sex, in each parallel stratum, IRs were similar and higher in African and Caribbean-Americans compared to rates that were similarly lower in European and Mexican-Americans (Supplement Table 3). We also compared IRs for these four race-ethnicity groups by age (Figure 1) and found parallel patterns in the age-specific rates. Thus, for ease of interpretation and to increase statistical power and the precision of estimates related to other variables, we collapsed these four race/ethnic groups into two, deemed “African/Caribbean or AAs/CAs” and “European/Mexican or EAs/MAs” (see Supplement Table 4 and Supplement Figure 3 for sex and parental history-specific IRs and age and parental history-specific IRs, respectively, for these two combined race/ethnicity groups.)
Analyses of incidence rates (IRs) by calendar time and place
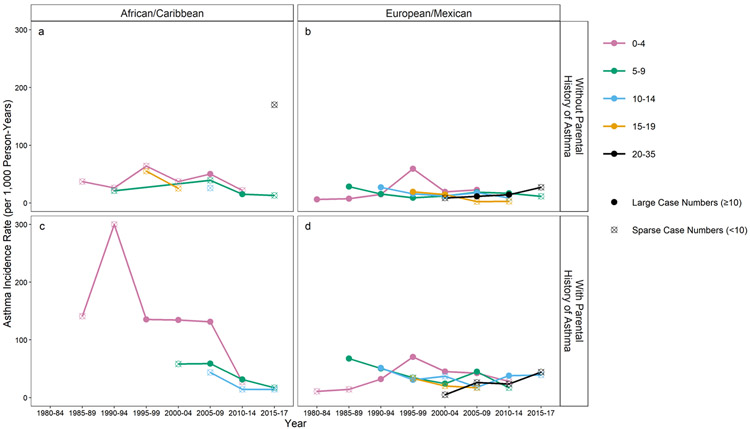
The first step in analyzing surveillance data is usually evaluating incidence rate patterns by year and geography (Supplement Figures 4 and 5). Supplement Figure 4 shows that those with a parental history had higher rates over time for both race/ethnicity categories. Supplement Figure 5a-d shows IRs by Midwest versus Northwest versus Southwest over time, stratified by parental history and the two race/ethnicity categories. However, differences in age distribution of populations being compared impact interpretation if rates vary by age. In this “constructed” population, new birth cohorts were initiated as the years progressed, so the age distribution of the pooled cohort changed over the years. Therefore, age-specific rates are presented in Figure 2a-d, revealing different patterns by age and calendar year, race/ethnicity and parental history. The 0-4 age group showed high rates in 1995-99 that were consistent in all race/ethnicity categories and parental history groups. Among the EA/MAs, the 5-9- and 10–14-year-old group rates peaked earlier than the 0-4 age group and then declined (see also Supplement Figure 6a-b). Although unstable before 1995, incidence rates in the 0-4 year old age group are much higher in the AA/CAs with a parental history than in other groups. There is a suggestion among this AA/CA group that rates peaked in 1990-94, remained high with IRs over 130.0 from 1995-2009, but then decreased and were similar to the other race/ethnic categories after 2010. Thus the changes in incidence rates over calendar time have been greatest in the 0-4 year olds and highest from 1985-2009 for AA/CAs with a parental history. Detailed calendar time-decade of birth-age-sex-race/ethnicity-parental history-specific rates are provided in Supplement Table 5.
Figure 2.
Doctor-diagnosed asthma incidence rates per 1000 person-years by calendar time (years) and age at diagnosis and by race/ethnicity and parental history of asthma; n of population = 5035.
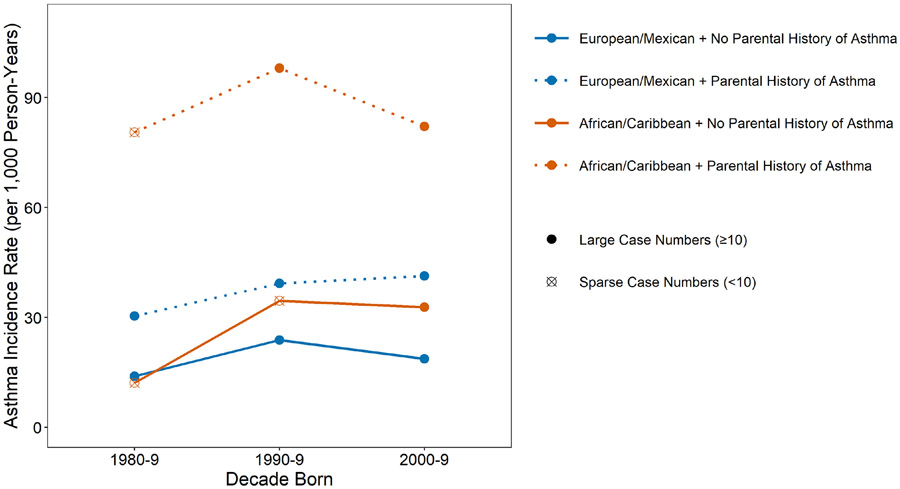
Analyses of incidence rates (IRs) by birth decade
IRs were examined next by decade of birth, race/ethnicity and parental history (Figure 3). While the IRs by race were similar in the 1980-1989 decade among those without a parental history, they diverged in the two subsequent birth decades, with higher IRs for AAs/CAs. For those with a parental history, the IRs for AA/CA were markedly higher than those for EA/MA were in all three birth decades examined. However, the IRs for EA/MAs with a positive parental history increased by birth decade, while those of the AA/CA peaked in the 1990-99 decade.
Figure 3.
Doctor-diagnosed asthma incidence rates per 1000 person-years by decade of birth and by race/ethnicity and parental history of asthma; n of population of 5035.
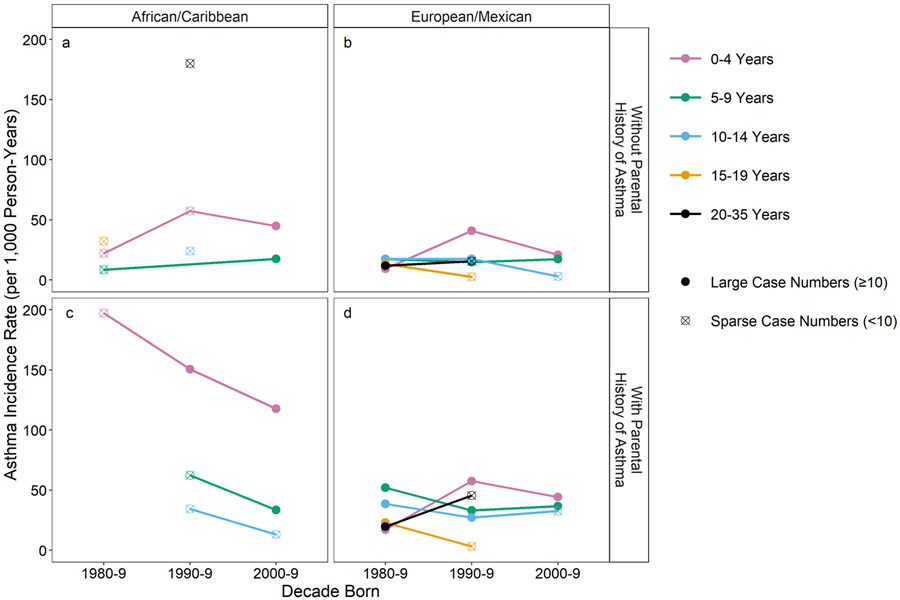
Considering age, the most striking changes in rates by decade of birth were found in the 0-4 year age groups (Figure 4). Among those without a parental history, 0-4 year-olds from both race/ethnicity groups born in the 1990s had the highest IRs compared to the two other decades and all other age groups (Figure 4a-b). Among those with a parental history, the EA/MA 0-4 year-olds again had the highest IRs during 1990-9, but the AA/CA 0-4 year-olds had higher IRs in 1980-1989 and dramatically declined in the two following decades, although fewer AA/CAs were enrolled in the 1980’s among the studied cohorts, impairing the precision of the IR estimates for this decade. Nevertheless, the rate for AA/CA 0-4 year olds born in the 1990s was striking at 150.5 (95% CI 107.5-204.9) per 1000 person-years, nearly three times the rate for EA/MA’s of the same age (57.6 (95% CI 45.9-71.3) per 1000 person-years).
Figure 4.
Doctor-diagnosed asthma incidence rates per 1000 person-years by decade of birth and age at diagnosis and by race/ethnicity and parental history of asthma; n of population of 5035.
Analyses of incidence rates (IRs) by age, race and sex
Differences in asthma patterns by sex and age have been a continuing scientific interest (Figure 5a-d). In subjects without a parental history (Fig 5 a-b) among the EA/MA, the female rates slightly increase from 0-4 to 10-14 years and then plateau. Male rates decline from 0-4 to 15-19 crossing female rates during the 10-14 year interval. In contrast, the rates for AA/CAs were highest in 0-4 year old children for both males and females, and while data are sparse, trends suggest that the rates initially decline from 0-4 years but then increased in males after 9 years and in females after 14 years. In those with a parental history (Fig 5 c-d), the rates are exceedingly high in 0-4 year old AA/CA males at ~150/1000 person-years and also high in AA/CA females of this age group (~100/1000 person-years) after which they rapidly decline but do not cross each other. In the EA/MAs with a parental history, there is a crossover in incidence by sex at an older age than found in those without a parental history and predominately reflects a more rapid decrease in the male rates rather than an increase in rates among females.
Figure 5.
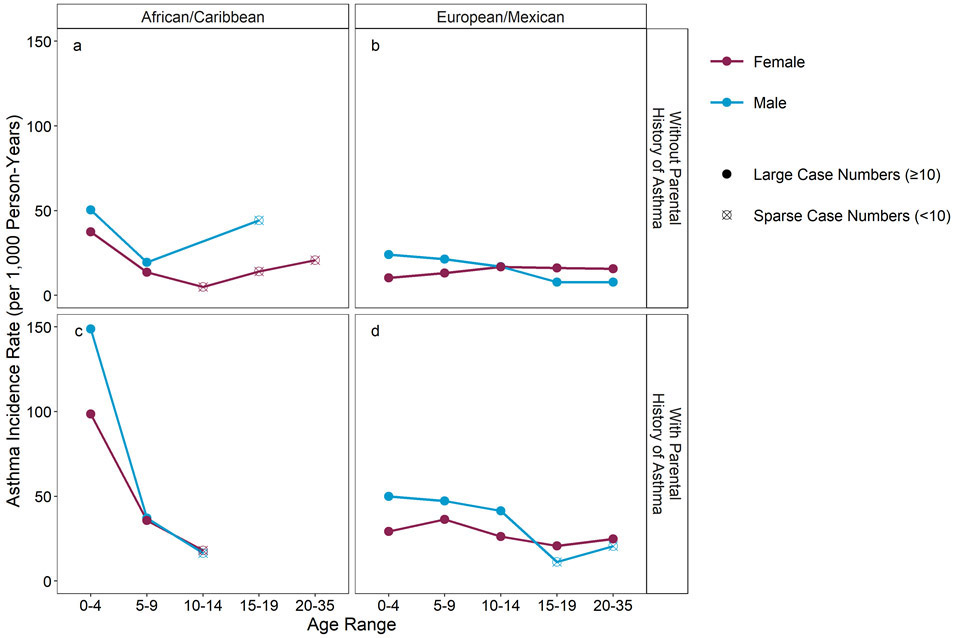
Doctor-diagnosed asthma incidence rates per 1000 person-years by age and child sex and by race/ethnicity and parental history of asthma; n of population of 5034.
Multivariable Analyses
Adjusted incidence rate ratios (aIRRs) were calculated using Poisson models to account for potential confounders and assess effect modification. Table 2, Model 1, displays aIRRs for the total population demonstrating that being born in the 1990s, younger, AA/CA, male, and having a parental history of asthma are all significantly associated (p< 0.001) with higher aIRRs of asthma. A sex by race/ethnicity term in this model was not significant, p=0.856 nor was the sex by family history term, p=0.496. However, since the parental history by race/ethnicity interaction term was significant at p=0.025, Models 2 and 3 assess effect modification by parental history. AA/CA race/ethnicity is a stronger risk factor in those with a parental asthma history (aIRR=2.16; CI 1.76-2.64) whereas the 1990s and 2000s as higher incidence birth decades seems more important among individuals without a parental history. The rate increases in the 0-4 and 5-9 age groups were stronger in those with a parental history. Models 4 and 5 stratify by decade of birth since the decade born by region interaction term was significant at p<0.001, not unexpected as there were few AA/CAs and northeastern US cohorts born in the 1980s compared to the 1990s and 2000s. For those born in the 1980s, the 5-9 yr. and 10-14 yr. old groups had the highest rates, rates in the Southwest region were higher than in the Midwest, and the aIRR for parental history was 2.19 (95%CI 1.81-2.65). For those born from 1990-2009, the aIRR was notably elevated for the 0-4 year olds (aIRR=5.08; 95%CI 2.77-9.30), rates were significantly lower in the southwest, males and AA/CAs had increased rates while parental history maintained a similar association.
Table 2.
Adjusted* Incidence Rate Ratios (aIRR) of doctor-diagnosed asthma by Poisson regression models, total and stratified by parental history and birth decade
| Model 1** All (N=5,034) |
Model 2 Without Parental History (N=3,185) |
Model 3 With Parental History (N=1,849) |
Model 4 Born 1980- 1989 (N=1,783) |
Model 5 Born 1990- 2009 (N=3,251) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | aIRR (95% CI) |
p- value |
aIRR (95% CI) |
p- value |
aIRR (95% CI) |
p- value |
aIRR (95% CI) |
p- value |
aIRR (95% CI) |
p- value |
| Decade Born | ||||||||||
| 2000-2009 | 1.13 (0.95, 1.33) |
0.168 | 1.29 (1.03, 1.62) |
0.028 | 0.94 (0.72, 1.23) |
0.670 | -- | -- | -- | -- |
| 1990-1999 | 1.32 (1.11, 1.59) |
0.002 | 1.46 (1.09, 1.96) |
0.012 | 1.16 (0.89, 1.51) |
0.274 | -- | -- | -- | -- |
| 1980-1989 | --reference-- | --reference-- | --reference-- | -- | -- | -- | -- | |||
| Region of United States | ||||||||||
| Northeast | 1.07 (0.88, 1.29) |
0.496 | 1.18 (0.86, 1.62) |
0.303 | 1.05 (0.80, 1.40) |
0.706 | *** | -- | 1.86 (1.39, 2.48) |
<0.001 |
| Midwest | 0.99 (0.85, 1.14) |
0.839 | 0.96 (0.80, 1.16) |
0.690 | 1.06 (0.83, 1.35) |
0.621 | 0.76 (0.62, 0.92) |
0.006 | 1.51 (1.15, 2.00) |
0.003 |
| Southwest | --reference-- | --reference-- | --reference-- | --reference-- | --reference-- | |||||
| Age group | ||||||||||
| 0-4 | 1.83 (1.51, 2.23) |
<0.001 | 1.42 (1.11, 1.82) |
0.005 | 2.68 (1.87, 3.84) |
<0.001 | 0.84 (0.65, 1.10) |
0.216 | 5.08 (2.77, 9.30) |
<0.001 |
| 5-9 | 1.24: (1.01, 1.53) |
0.039 | 1.14 (0.88, 1.47) |
0.333 | 1.61 (1.11, 2.35) |
0.012 | 1.73 (1.37, 2.18) |
<0.001 | 2.32 (1.25, 4.29) |
0.007 |
| 10-14 | 1.18 (0.94, 1.48) |
0.149 | 1.18 (0.90, 1.56) |
0.227 | 1.33 (0.88, 2.01) |
0.173 | 1.56 (1.21, 2.00) |
0.001 | 1.71 (0.89, 3.30) |
0.106 |
| 15-35 | --reference-- | --reference-- | --reference-- | --reference-- | --reference-- | |||||
| Race/Ethnicity | ||||||||||
| African/Caribbean American | 1.89 (1.62, 2.20) |
<0.001 | 1.56 (1.24, 1.98) |
<0.001 | 2.16 (1.76, 2.64) |
<0.001 | 1.17 (0.64, 2.13) |
0.610 | 1.58 (1.38, 1.80) |
<0.001 |
| European/Mexican American | --reference-- | --reference-- | --reference-- | --reference-- | --reference-- | |||||
| Sex | ||||||||||
| Male | 1.39 (1.25, 1.54) |
<0.001 | 1.33 (1.15, 1.55) |
<0.001 | 1.43 (1.24, 1.65) |
<0.001 | 1.14 (0.96, 1.36) |
0.136 | 1.52 (1.33, 1.73) |
<0.001 |
| Female | --reference-- | --reference-- | --reference-- | --reference-- | --reference-- | |||||
| Parental Asthma History | ||||||||||
| Yes | 2.16 (1.93, 2.42) |
<0.001 | -- | -- | -- | -- | 2.19 (1.81, 2.65) |
<0.001 | 2.11 (1.83, 2.42) |
<0.001 |
| No | --reference-- | -- | -- | -- | -- | --reference-- | --reference-- |
In each model, all variables adjusted for all the other variables in the model.
For Model 1, the aIRR of the 2000-2009 decade as compared to 1990-1999 decade is 1.10 (0.94, 1.29); p=0.220
Indicates there were insufficient numbers to permit an estimate.
Discussion
The incidence rate, defined as the number of new cases of a disease over a specified time period occurring in a defined population at risk for the disease, is a fundamental measure in epidemiology because incidence rates are critical for understanding disease etiology and causality.47 Other epidemiological measures, such as mortality rates and prevalence ratios, are less valuable in relationship to causality because many factors beyond etiology come into play with these estimates. Measuring childhood asthma incidence rates over a nearly 40-year period, we found that the rates revealed marked differences over calendar time, and by age, racial/ethnic background, sex and parental asthma history.
The most highly cited study providing incidence rates for pediatric asthma in the United States was published in 1992 by Yunginger and colleagues from the Mayo Clinic, covering the period 1964-1983.7 The population was 97% white and living in a county of only about 100,000 residents in 1980 and lacked information on parental history of asthma. Nevertheless, this landmark study suggested that the incidence of asthma was increasing in the period just preceding when our data collection was initiated, with rates in the early 1980s comparable to those found in our EA/MA population at that time. In addition, the largest increases over calendar time were in children 0-14, particularly in those from 1-4 years of age, similar to our findings (see Supplement Figures 6a-b for CREW European-American only IRs by calendar time and age comparable to those found in the Yunginger et al.7 publication). The peak rates for 1-4 year old children in 1983 in Olmstead County was ~10 per 1000, compared to 6.8 per 1000 person-years in 1980-84 for our EA/MA group, which consisted of children from the earliest Tucson-based cohort. They also saw differential incidence rates over time for males and females depending upon age, with the increase in incidence over time greater in males aged 1-9 years (and especially 1-4 years old), but not in older age groups.
Many questions have been raised regarding the effects of race and ethnicity on epidemiological and clinical aspects of pediatric asthma. Among the cohorts analyzed in this study, there were minimal differences in incidence rates among those considering themselves as Americans of predominately European origins and those considering themselves as Americans of Mexican origin. Similarly, there were minimal differences among those considering themselves as African-Americans and those identifying Caribbean heritage, in this study largely children of Dominican or Puerto Rican origins. These findings differ from several studies of prevalence reporting that among children, asthma prevalence is highest among Puerto Ricans, followed by blacks, whites and lowest among Hispanics of Mexican heritage.39, 48-52 The apparent differences between the incidence and prevalence findings could be related to variation in remission rates. Some long term cohort studies have shown that asthma is variably present through life with more remissions during childhood,53-55 but there is little information on whether asthma remission varies among racial/ethnic groups.56 Hispanic populations from the Caribbean, and specifically Puerto Rico and the Dominican Republic, have been shown to have a large percentage of African ancestry, whereas Mexican-Americans do not.57, 58 These findings along with the very high incidence rates in the youngest age group of AA/CA children with parental histories of asthma, and the minimal geographic variation, suggest that there may be a factor associated with African ancestry that increases the risk of asthma inception.59 However, while genetics likely plays a role, numerous non-genetic factors associated with race/ethnicity in the US over multiple generations appear critically important and certainly contribute to the observation that positive asthma family history is a potent influence on incidence rates. Moreover, the dramatic changes in asthma incidence rates over just a few decades that vary by race/ethnicity suggest environmental exposures and social circumstances contribute substantially to disease risk.60
Important questions arising from monitoring asthma prevalence is the increase in prevalence during the 1990’s and 2000’s followed by an apparent plateau or decline afterwards. Our data show a marked decline in the incidence rates of asthma from the 2005-2009 period to 2010-2014 among AA/CA children with parental histories of asthma, which occurred similarly in the three areas of the US considered. This change was predominately among children less than 4 years of age. The declining incidence in these years is also clearly seen among AA/CA without a parental history, and to a lesser degree in the other race/ethnicity-parental history groups. Among EA/MAs the peak incidence rates in 0-4 year olds was from 1995-99 but was earlier for the 5-9 year group (1985-1989). A study by Engelkes M. et al. examined asthma incidence rates among 5-18 year old children in the Netherlands from 1999 to 2012 and reported an apparent decline from 2007 to 2012 with a pivot point of 2008-2009, supporting our findings.20 Another study of asthma incidence rates in Denmark and Sweden showed an increase in asthma incidence from 1998 to 2006 followed by a similar decline to 2011 in Danish children but, in contrast, an increase in incidence from 2006 to 2010 in Swedish children.21 Simpson et al. in England61 reported a decline in asthma incidence in all age groups from birth to 65+ years from 2001 to 2005, and especially in 2005; similarly, the largest declines were also in children ages 0-4 years (−38.4%) as well as 5-14 years (−27.0%).
The pattern of uptick and then decline in asthma incidence rates over time and by decade born suggests that one or more novel external factors were increasingly introduced in the 1980s and more dramatically influenced 0-4 year old children and all race groups, but especially those with African heritage and those with a parental history of asthma. This factor may have then changed in a way that substantially reduced the number of new childhood asthma diagnoses both in Europe and across the US, with the decline first witnessed among U.S. children of European origin. It remains for investigators to consider and evaluate possible reasons for such a change. A strong candidate for one such factor is the changing use of antibiotics during early infancy in response to antimicrobial stewardship promotion, an exposure that has been examined in the past with respect to risk for allergies and asthma with conflicting results.62-64 In a 2020 comprehensive paper, Patrick et al. reported that antibiotic prescribing in children less than a year old had declined 77% from 1996-2016 in the province of British Columbia, Canada, according to health care administrative data, and from 2000-2014 this decline was associated with a significant decrease in asthma diagnoses in children from age 1-4 years old.65 Moreover, in the Canadian CHILD cohort, the use of systemic antibiotics before 1 year of age was associated with significantly increased risk for an asthma diagnosis at age 5 years, which was shown to be mediated by the gut microbial community at age 1 year. Interestingly, a U.S. study of claims data across nine health plans showed a significant decrease (24%) in antibiotic dispensed medications in children aged <3 months-<3 years between 1996 and 2000.66 Further, over this period of time, many other exposures potentially related to the infant gut microbial community and/or childhood asthma risk have changed, such as types and quantities of air pollution, chemicals in foods and on clothing, microbes in food, decreased early infections and changes in pediatric vaccines, medically-based campaigns to alter infant care, drinking water quality, and physical activity levels.67, 68
One of the more consistent findings in prevalence data is a decline in the prevalence of asthma in males during the mid-teenage years and an increase in prevalence among females. Many have assumed that this change resulted from an increase in incidence rates among females related to puberty. However, our data do not show a large increase in incidence rates among females approaching the teenage years. Among AA/CA the incidence for males is always greater or the same as females, although our data were limited for AA/CA in the older age groups. Among the EA/MA, the incidence rates for males and females do cross between 10 and 19 years for those with and without a parental history, but the change in incidence rates is primarily because of a rapid decline in the incidence rates in males after the first decade with little change in females. This same pattern of a higher male incidence declining rapidly between 10 and 20 years of age to fall below the incidence for females has been reported by others.20, 22, 28 It has been suggested that older females may be more likely to develop non-IgE-related asthma, which would then be reflected in overall asthma age-specific incidence rates and prevalence, highlighting the need for more precise asthma phenotyping in epidemiological studies.69, 70
Strengths of our study include the large, racially/ethnically and geographically diverse cohorts recruited over more than 30 years who were specifically followed frequently and queried about allergic diseases and asthma. The pooling and harmonization of data from these nine cohorts allowed relatively robust estimates of asthma incidence rates over three decades while considering the child’s age, race/ethnicity, sex, and region of birth within the United States as well as parental history. While it is known that the incidence of an asthma diagnosis among children 0-4 years of age may often be a transient wheezing phenotype,71 our intent was to evaluate asthma incidence rates as they would appear if an asthma diagnosis was a reportable event as part of a routine surveillance system covering all asthma diagnoses and therefore all phenotypes. However, it is reassuring that our results do not conflict with the ECHO-wide incidence study mentioned above,14 in which children with a diagnosis of asthma or wheeze before the age of 5 years were not considered an incident case at that time point unless there also was sustained evidence of disease after the fifth birthday.
As with all studies, ours had important limitations. There were minor variations between studies in the specific questions asked about asthma diagnosis that had to be reconciled by expert opinion as the data were harmonized. Because these were longitudinal studies of large populations, there were inevitable losses to follow up in each cohort. Similarly, missing data reduced the numbers of subjects for certain analyses. Just US populations were considered and were restricted to only 8 states. Determinations of race and ethnicity were dependent upon the parental reports rather than on ancestral DNA markers. As already mentioned, the racial/ethnic makeup of cohorts and their selection criteria varied over the decades of recruitment, making early period estimates of incidence rates for minority groups less robust. It is also impossible to ascertain how changes in the diagnostic criteria for asthma over time, by racial group or by region of the country affected our results. However, it is unlikely that national or local diagnostic trends would have systematically influenced only certain subgroups, particularly by age, as was revealed by our data, suggesting that this may be less of a concern. It would have been valuable to have information on other variables such as allergic sensitization and sequential spirometry to provide more specific phenotypes, but this awaits future analyses, as these data, which are also less widely available, have not yet been harmonized.
In summary, we found that asthma incidence rates varied dramatically by age, sex, racial/ethnic background and parental asthma history. The highest asthma incidence rates were among AA/CA children 0-4 years of age, especially males, who had parental histories of asthma. We found that these variables affected rates in both males and females, but especially pre-school males. Asthma incidence rates also varied by decade of birth with a peak in 1900’s births. The increase and relatively high incidence rates for asthma followed by declines suggest that it would be valuable to search for environmental and behavioral changes occurring during the relevant time-periods. Importantly, our findings illustrate the potential value of a U.S. asthma surveillance system for discerning patterns and generating etiological hypotheses that could lead to disease prevention.
Supplementary Material
Key Messages.
Childhood asthma incidence rates in the United States have varied dramatically by age, racial/ethnic background, child sex and parental history.
Asthma incidence rates were highest in 0-4 year olds, particularly AA/CA males with a parental history, but rates for this race/ethnic category declined after 2009. A defined peak for EA/MA 0-4 year olds was in 1995-1999.
Funding:
The research reported in this publication was supported by the CREW consortium, part of NIH Environmental influences on Child Health Outcomes (ECHO). CREW is funded by HHS/NIH grant 5UG3OD023282. Additional support was provided by individual cohorts’ grants/contracts:
Columbia University: P01ES09600, R01 ES008977, P30ES09089, R01 ES013163, R827027
Tucson Children’s Respiratory Study (TCRS): NHLBI 132523
Infant Immune Study (IIS): HL-56177
Childhood Origins of ASThma Study (COAST): P01 HL070831, U10 HL064305, R01 HL061879.
Urban Environment and Childhood Asthma Study (URECA): NO1-AI-25496, NO1-AI-25482, HHSN272200900052C, HHSN272201000052I, 1UM1AI114271-01, UM2AI117870, NCRR/NIH RR00052, M01RR00533, 1UL1RR025771, M01RR00071, 1UL1RR024156, UL1TR001079, 5UL1RR024992-02, NCATS/NIH UL1TR000040.
Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS): R01 ES11170, R01 ES019890
The Epidemiology of Home Allergens and Asthma Study (EHAAS):. R01 AI035786
Wayne County Health, Environment, Allergy and Asthma Longitudinal Study (WHEALS): R01 AI050681, R56 AI050681, R01 AI061774, R21 AI059415, K01 AI070606, R21 AI069271, R01 HL113010, R21 ES022321, P01 AI089473, R21 AI080066, R01 AI110450, R01 HD082147, and the Fund for Henry Ford Hospital.
Childhood Allergy Study (CAS): R01 AI024156; R03 HL067427, R01 AI051598, Blue Cross Foundation, and the Fund for Henry Ford Hospital
ACKNOWLEDGMENTS
The Children’s Environment and Respiratory Workgroup (CREW) would like to acknowledge the following institutions, investigators and staff whose efforts contributed to the work presented in this manuscript (principal investigators are indicated by an asterisk):
Columbia Center for Children’s Environmental Health (CCCEH): Rachel Miller*, Howard Andrews, Julie Herbstman, Lori Hoepner, Frederica Perera, Matthew Perzanowski, Xinhua Liu, Judyth Ramirez, Janelle Rivera, Deliang Tang, Kylie Wheelock Riley, Jacqueline Jezioro, Lydia Lichtiger, Teresa Durham, Diurka Diaz, Gladys Badia.
Tucson Children’s Respiratory Study (TCRS): Anne L. Wright*, Fernando D. Martinez*, Wayne Morgan, Debra A. Stern, Dean Billheimer, Brian Hallmark, Paloma Beamer, Nathan Lothrop, Lydia De La Ossa, Silvia Lopez, Marilyn Halonen, Amber Spangenberg, David Spies.
Infant Immune Study (IIS): Anne L. Wright*, Fernando D. Martinez*, Wayne Morgan, Debra A. Stern, Dean Billheimer, Brian Hallmark, Paloma Beamer, Nathan Lothrop, Heidi Erickson, Marilyn Halonen, Amber Spangenberg, David Spies
Childhood Origins of Asthma Study (COAST): Robert F. Lemanske, Jr.*, Daniel J. Jackson*, James E. Gern, Carole Ober, Anne Marie Singh, Ronald E. Gangnon, Michael D. Evans, Victoria Rajamanickam, Christopher Tisler, Lisa Salazar, Susan Doyle, Yury Bochkov, Rebecca Brockman-Schneider, Rose Vrtis, Kristine Grindle, Tressa Pappas, Elizabeth Anderson, Kathy Roberg, Kirsten Carlson-Dakes, Mark DeVries, Douglas DaSilva, Ronald Sorkness, Lance Mikus, Julia Bach.
Urban Environment and Childhood Asthma Study (URECA):
Johns Hopkins University, Baltimore, MD: R Wood*, E Matsui, H Lederman, F Witter, S Leimenstoll, D Scott, M Cootauco, P Jones; Boston University School of Medicine, Boston, MA: G O’Connor*, W Cruikshank, M Sandel, A Lee-Parritz, C Jordan, E Gjerasi, P Price-Johnson, L Gagalis, L Wang, N Gonzalez, M Tuzova; Harvard Medical School, Boston, MA – D Gold, R Wright; Columbia University, New York, NY: M Kattan*, C Lamm, N Whitney, P Yaniv, M Pierce, J Jezioro; Mount Sinai School of Medicine, New York, NY: H Sampson, R Sperling, N Rivers; Washington University School of Medicine, St Louis, MO: G Bloomberg*, L Bacharier*, Y Sadovsky, E Tesson, C Koerkenmeier, R Sharp, K Ray, J Durrange, I Bauer, A Freie, V Morgan; Statistical and Clinical Coordinating Center - Rho, Inc, Chapel Hill, NC: C Visness*, P Zook, M Yaeger, J Martin, A Calatroni, K Jaffee, W Taylor, R Budrevich, H Mitchell; Scientific Coordination and Administrative Center, University of Wisconsin, Madison, WI: W Busse*, J Gern*, P Heinritz, C Sorkness, K. Hernandez, Y. Bochkov, K Grindle, A Dresen, T Pappas, M. Renneberg, B. Stoffel; National Institute of Allergy and Infectious Diseases, Bethesda, MD: P Gergen, A Togias, E Smartt, K Thompson.
Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS): Gurjit K. Khurana Hershey*, Patrick H. Ryan*, Jocelyn M. Biagini Myers*, Lisa J. Martin*, Grace K. LeMasters*, Kristi Curtsinger, Liza Bronner Murrison*, Jeffrey W. Burkle, Christopher Wolfe, Zachary Flege, David Morgan, Kristina Keidel, Taylor Groeschen, Jessica Riley.
The Epidemiology of Home Allergens and Asthma Study (EHAAS): Diane R. Gold, Soma Datta, Sharon O’Toole, Conner Fleurat, Leanna Farnham.
Wayne County Health, Environment, Allergy and Asthma Longitudinal Study (WHEALS):
Henry Ford Health System, Detroit, MI: CC Johnson*, G Wegienka, S Havstad, E Zoratti, A Cassidy-Bushrow, A Levin, H Kim, K Woodcroft, A Sitarik, C Joseph, LK Williams, C Barone, K Bobbitt, S Zhang, J Campbell, K Bourgeois, M Aubuchon, J Ezell, K Jones; Augusta University, Augusta, GA: D Ownby
Childhood Allergy Study (CAS): D Ownby*, CC Johnson, C Joseph, E Zoratti, G Wegienka, S Havstad, K Woodcroft, E Peterson, S Hensley Alford, J McCullough, C Strauchman Boyer, S Blocki, G Birg, N Akkerman, K Wells, S Zhang, C Nicholas, A Jones, G Stouffer
Administrative Center, University of Wisconsin-Madison: James E. Gern,* Gina Crisafi, Dorothy Floerke, Rick Kelley.
Coordinating Center, Rho, Inc. Federal Systems Division: Cynthia Visness*, Melissa Yaeger, Samara Dixon, Jessica Baucom, Caitlin Suddeuth.
Biomedical Informatics and Biostatistical Core (BBC), University of Wisconsin-Madison: Umberto Tachinardi*, Mark Craven*, Eneida Mendonca, Lisa Gress, Adam Nunez, Laura Ladick
University of Manchester: Philip Couch, Camille Johnson, John Ainsworth, Victoria Turner
Imperial College, London UK: Adnan Custovic
Footnotes
This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. None of the authors reports a conflict of interest.
References
- 1.Bonita R, Beaglehole R, Kjellstrom T. Basic Epidemiology. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 2.MacMahon B, Pugh TF. Epidemiology: Principles and Methods. Boston, MA: Little, Brown and Company; 1970. [Google Scholar]
- 3.https://www.cdc.gov/nndss/conditions/notifiable/2019.].
- 4.Broder I, Higgins MW, Mathews KP, Keller JB. Epidemiology of asthma and allergic rhinitis in a total community, tecumseh, Michigan.IV . Natural history. Journal of Allergy and Clinical Immunology 1974; 54:100–10. [DOI] [PubMed] [Google Scholar]
- 5.Broder I, Higgins MW, Mathews KP, Keller JB. Epidemiology of asthma and allergic rhinitis in a total community, Tecumseh, Michigan. 3. Second survey of the community. Journal of Allergy and Clinical Immunology 1974; 53:127–38. [DOI] [PubMed] [Google Scholar]
- 6.Dodge RR, Burrows B. The prevalence and incidence of asthma and asthma-like symptoms in a general population sample. Am Rev Respir Dis 1980; 122:567–75. [DOI] [PubMed] [Google Scholar]
- 7.Yunginger JW, Reed CE, O'Connell EJ, Melton LJ 3rd, O'Fallon WM, Silverstein MD. A community-based study of the epidemiology of asthma. Incidence rates, 1964-1983. Am Rev Respir Dis 1992; 146:888–94. [DOI] [PubMed] [Google Scholar]
- 8.de Marco R, Locatelli F, Cazzoletti L, Bugianio M, Carosso A, Marinoni A. Incidence of asthma and mortality in a cohort of young adults: a 7-year prospective study. Respir Res 2005; 6:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeatts K, Sly P, Shore S, Weiss S, Martinez F, Geller A, et al. A brief targeted review of susceptibility factors, environmental exposures, asthma incidence, and recommendations for future asthma incidence research. Environ Health Perspect 2006; 114:634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trepka MJ, Martin P, Mavunda K, Rodriguez D, Zhang G, Brown C. A pilot asthma incidence surveillance system and case definition: lessons learned. Public Health Rep 2009; 124:267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koutsavlis AT, Kosatsky T, Cox J, Goyer E. Reporting childhood asthma: why? Why not? What else? J Public Health Policy 2001; 22:311–9. [PubMed] [Google Scholar]
- 12.https://seer.cancer.gov/registries/.].
- 13.Jajosky R, Rey A, Park M, Aranas A, Macdonald S, Ferland L. Findings from the Council of State and Territorial Epidemiologists' 2008 assessment of state reportable and nationally notifiable conditions in the United States and considerations for the future. J Public Health Manag Pract 2011; 17:255–64. [DOI] [PubMed] [Google Scholar]
- 14.Johnson CC, Chandran A, Havstad S, Li X, McEvoy C, Ownby D, et al. Childhood asthma incidence rate patterns from the ECHO Consortium: Identifying high risk groups for primary prevention. JAMA Peds 2021, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correia de Sousa J, Silva ML, Lobo FA, Yaphe J. Asthma incidence and accuracy of diagnosis in the Portuguese sentinel practice network. Prim Care Respir J 2010; 19:352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gershon AS, Guan J, Wang C, To T. Trends in asthma prevalence and incidence in Ontario, Canada, 1996-2005: a population study. Am J Epidemiol 2010; 172:728–36. [DOI] [PubMed] [Google Scholar]
- 17.Wendt JK, Symanski E, Du XL. Estimation of asthma incidence among low-income children in Texas: a novel approach using Medicaid claims data. Am J Epidemiol 2012; 176:744–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.To T, Stanojevic S, Feldman R, Moineddin R, Atenafu EG, Guan J, et al. Is asthma a vanishing disease? A study to forecast the burden of asthma in 2022. BMC Public Health 2013; 13:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawson JA, Janssen I, Bruner MW, Hossain A, Pickett W. Asthma incidence and risk factors in a national longitudinal sample of adolescent Canadians: a prospective cohort study. BMC Pulm Med 2014; 14:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelkes M, Janssens HM, de Ridder MA, de Jongste JC, Sturkenboom MC, Verhamme KM. Time trends in the incidence, prevalence and age at diagnosis of asthma in children. Pediatr Allergy Immunol 2015; 26:367–74. [DOI] [PubMed] [Google Scholar]
- 21.Henriksen L, Simonsen J, Haerskjold A, Linder M, Kieler H, Thomsen SF, et al. Incidence rates of atopic dermatitis, asthma, and allergic rhinoconjunctivitis in Danish and Swedish children. J Allergy Clin Immunol 2015; 136:360–6.e2. [DOI] [PubMed] [Google Scholar]
- 22.Bardal S, Smith A, Luo HA, Zhang T, Groeneweg G, Jimenez Mendez R, et al. Asthma in British Columbia: Are we finally breathing easier? A population-based study of the burden of disease over 14 years. J Asthma 2017; 54:308–17. [DOI] [PubMed] [Google Scholar]
- 23.Rönmark E, Perzanowski M, Platts-Mills T, Lundbäck B. Incidence rates and risk factors for asthma among school children: a 2-year follow-up report from the obstructive lung disease in Northern Sweden (OLIN) studies. Respir Med 2002; 96:1006–13. [DOI] [PubMed] [Google Scholar]
- 24.Rudd RA, Moorman JE. Asthma incidence: data from the National Health Interview Survey, 1980-1996. J Asthma 2007; 44:65–70. [DOI] [PubMed] [Google Scholar]
- 25.Winer RA, Qin X, Harrington T, Moorman J, Zahran H. Asthma incidence among children and adults: findings from the Behavioral Risk Factor Surveillance System asthma call-back survey--United States, 2006-2008. J Asthma 2012; 49:16–22. [DOI] [PubMed] [Google Scholar]
- 26.Black MH, Zhou H, Takayanagi M, Jacobsen SJ, Koebnick C. Increased asthma risk and asthma-related health care complications associated with childhood obesity. Am J Epidemiol 2013; 178:1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Marco R, Pattaro C, Locatelli F, Svanes C, Group ES. Influence of early life exposures on incidence and remission of asthma throughout life. J Allergy Clin Immunol 2004; 113:845–52. [DOI] [PubMed] [Google Scholar]
- 28.Honkamäki J, Hisinger-Mölkänen H, Ilmarinen P, Piirilä P, Tuomisto LE, Andersén H, et al. Age- and gender-specific incidence of new asthma diagnosis from childhood to late adulthood. Respir Med 2019; 154:56–62. [DOI] [PubMed] [Google Scholar]
- 29.Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ 1996; 312:1195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eagan TM, Bakke PS, Eide GE, Gulsvik A. Incidence of asthma and respiratory symptoms by sex, age and smoking in a community study. Eur Respir J 2002; 19:599–605. [DOI] [PubMed] [Google Scholar]
- 31.Pereira Vega A, Sanchez Ramos JL, Maldonado Perez JA, Sanchez Rodriguez I, Gil Munoz FL, Garcia Jimenez D. [Asthma incidence in Huelva, Spain at 2 stages of life: childhood and young adulthood]. Arch Bronconeumol 2008; 44:464–70. [PubMed] [Google Scholar]
- 32.Broms K, Norback D, Sundelin C, Eriksson M, Svardsudd K. A nationwide study of asthma incidence rate and its determinants in Swedish pre-school children. Eur J Epidemiol 2012; 27:695–703. [DOI] [PubMed] [Google Scholar]
- 33.Gergen PJ, Togias A. Inner city asthma. Immunol Allergy Clin North Am 2015; 35:101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN. Vital Signs: Asthma in Children - United States, 2001-2016. MMWR Morb Mortal Wkly Rep 2018; 67:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter-Pokras OD, Gergen PJ. Reported asthma among Puerto Rican, Mexican-American, and Cuban children, 1982 through 1984. Am J Public Health 1993; 83:580–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keet CA, McCormack MC, Pollack CE, Peng RD, McGowan E, Matsui EC. Neighborhood poverty, urban residence, race/ethnicity, and asthma: Rethinking the inner-city asthma epidemic. J Allergy Clin Immunol 2015; 135:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah R, Newcomb DC. Sex bias in asthma prevalence and pathogenesis. Front Immunol 2018; 9:2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson HR, Gupta R, Strachan DP, Limb ES. 50 years of asthma: UK trends from 1955 to 2004. Thorax 2007; 62:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, et al. National surveillance for asthma--United States, 1980-2004. Morbidity & Mortality Weekly Report.Surveillance Summaries.56(8):1–54, 2007. [PubMed] [Google Scholar]
- 40.Anandan C, Nurmatov U, van Schayck OC, Sheikh A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy 2010; 65:152–67. [DOI] [PubMed] [Google Scholar]
- 41.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980-2007. Pediatrics.123 Suppl 3:S131–45, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Brozek G, Lawson J, Szumilas D, Zejda J. Increasing prevalence of asthma, respiratory symptoms, and allergic diseases: Four repeated surveys from 1993-2014. Respir Med 2015; 109:982–90. [DOI] [PubMed] [Google Scholar]
- 43.Gern JE, Jackson DJ, Lemanske RF, Seroogy CM, Tachinardi U, Craven M, et al. The Children's Respiratory and Environmental Workgroup (CREW) birth cohort consortium: design, methods, and study population. Respir Res 2019; 20:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pividori M, Schoettler N, Nicolae DL, Ober C, Im HK. Shared and distinct genetic risk factors for childhood-onset and adult-onset asthma: genome-wide and transcriptome-wide studies. Lancet Respir Med 2019; 7:509–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huber P The behavior of maximum likelihood estimates under nonstandard conditions. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. Berkeley, CA: University of California Press, 1967:221–33. [Google Scholar]
- 46.White H A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica 1980; 48:817–30. [Google Scholar]
- 47.Rothman K, Greenland S. Modern Epidemiology. 2nd ed: Lippincott-Raven Publishers;; 1998. [Google Scholar]
- 48.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005-2009. National health statistics reports.(32):1–14, 2011. [PubMed] [Google Scholar]
- 49.Akinbami LJ, Simon AE, Rossen LM. Changing trends in asthma prevalence among children. Pediatrics 2016; 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carr TF, Beamer PI, Rothers J, Stern DA, Gerald LB, Rosales CB, et al. Prevalence of asthma in school children on the arizona-sonora border. J Allergy Clin Immunol Pract 2017; 5:114–20.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Claudio L, Stingone JA, Godbold J. Prevalence of childhood asthma in urban communities: the impact of ethnicity and income. Ann Epidemiol 2006; 16:332–40. [DOI] [PubMed] [Google Scholar]
- 52.Lara M, Akinbami L, Flores G, Morgenstern H. Heterogeneity of childhood asthma among Hispanic children: Puerto Rican children bear a disproportionate burden. Pediatrics.117(1):43–53, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med 2003; 349:1414–22. [DOI] [PubMed] [Google Scholar]
- 54.Wang AL, Datta S, Weiss ST, Tantisira KG. Remission of persistent childhood asthma: Early predictors of adult outcomes. J Allergy Clin Immunol 2019; 143:1752–9.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andersson M, Hedman L, Bjerg A, Forsberg B, Lundback B, Ronmark E. Remission and persistence of asthma followed from 7 to 19 years of age. Pediatrics 2013; 132:e435–42. [DOI] [PubMed] [Google Scholar]
- 56.Javed A, Yoo KH, Agarwal K, Jacobson RM, Li X, Juhn YJ. Characteristics of children with asthma who achieved remission of asthma. J Asthma 2013; 50:472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Montinaro F, Busby GB, Pascali VL, Myers S, Hellenthal G, Capelli C. Unravelling the hidden ancestry of American admixed populations. Nat Commun 2015; 6:6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reibman J, Liu M. Genetics and asthma disease susceptibility in the US Latino population. Mt Sinai J Med 2010; 77:140–8. [DOI] [PubMed] [Google Scholar]
- 59.Daya M, Barnes KC. African American ancestry contribution to asthma and atopic dermatitis. Ann Allergy Asthma Immunol 2019; 122:456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsui EC, Adamson AS, Peng RD. Time's up to adopt a biopsychosocial model to address racial and ethnic disparities in asthma outcomes. J Allergy Clin Immunol 2019; 143:2024–5. [DOI] [PubMed] [Google Scholar]
- 61.Simpson CR, Sheikh A. Trends in the epidemiology of asthma in England: a national study of 333,294 patients. J R Soc Med 2010; 103:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson CC, Ownby DR, Alford SH, Havstad SL, Williams LK, Zoratti EM, et al. Antibiotic exposure in early infancy and risk for childhood atopy. J Allergy Clin Immunol 2005; 115:1218–24. [DOI] [PubMed] [Google Scholar]
- 63.Dharmage SC, Lodge CJ, Lowe AJ, Allen KJ. Antibiotics and risk of asthma: a debate that is set to continue. Clin Exp Allergy 2015; 45:6–8. [DOI] [PubMed] [Google Scholar]
- 64.Donovan BM, Abreo A, Ding T, Gebretsadik T, Turi KN, Yu C, et al. Dose, timing, and type of infant antibiotic use and the risk of childhood asthma. Clin Infect Dis 2020; 70:1658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patrick DM, Sbihi H, Dai DLY, Al Mamun A, Rasali D, Rose C, et al. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: evidence from population-based and prospective cohort studies. Lancet Respir Med 2020. [DOI] [PubMed] [Google Scholar]
- 66.Finkelstein JA, Stille C, Nordin J, Davis R, Raebel MA, Roblin D, et al. Reduction in antibiotic use among US children, 1996-2000. Pediatrics 2003; 112:620–7. [DOI] [PubMed] [Google Scholar]
- 67.Kravitz-Wirtz N, Teixeira S, Hajat A, Woo B, Crowder K, Takeuchi D. Early-life air pollution exposure, neighborhood poverty, and childhood asthma in the united states, 1990⁻2014. Int J Environ Res Public Health 2018; 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beasley R, Semprini A, Mitchell EA. Risk factors for asthma: is prevention possible? Lancet 2015; 386:1075–85. [DOI] [PubMed] [Google Scholar]
- 69.Keller T, Hohmann C, Standl M, Wijga AH, Gehring U, Melen E, et al. The sex-shift in single disease and multimorbid asthma and rhinitis during puberty - a study by MeDALL. Allergy 2018; 73:602–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hohmann C, Keller T, Gehring U, Wijga A, Standl M, Kull I, et al. Sex-specific incidence of asthma, rhinitis and respiratory multimorbidity before and after puberty onset: individual participant meta-analysis of five birth cohorts collaborating in MeDALL. BMJ Open Respir Res 2019; 6:e000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bacharier LB, Beigelman A, Calatroni A, Jackson DJ, Gergen PJ, O'Connor GT, et al. Longitudinal phenotypes of respiratory health in a high-risk urban birth cohort. Am J Respir Crit Care Med 2019; 199:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.