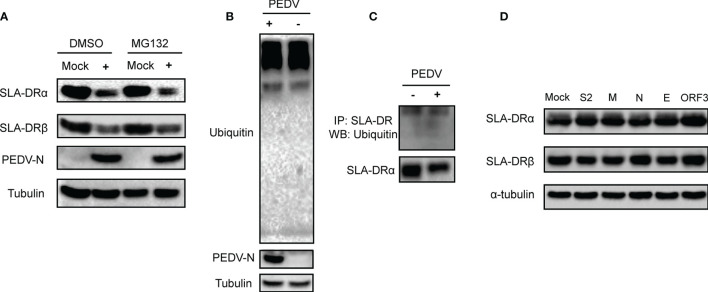
Figure 8.
Ubiquitin-proteasome pathway did not involve in inhibition of SLA-DR expression. (A) BM-DCs were infected by PEDV-KB2013-p120 strain at 1MOI for 24, then followed by treatment of either DMSO or MG132 for another 24 hours. Then cells were harvested for western blot evaluating of SLA-DRα, SLA-DRβ and PEDV-N protein. Normal BM-DCs cells without PEDV infection but treated with the same dose of DMSO or MG132 were included as controls. Tubulin was probed from the same sample to normalize the total protein load. (B) BM-DCs were infected by PEDV-KB2013-p120 strain at 1MOI for 24 hours. Next, cells were harvested for western blot evaluating of universal ubiquitination level and PEDV-N protein. Normal BM-DCs cells were included as control. Tubulin was probed from the same sample to normalize the total protein load. (C) BM-DCs were infected by PEDV-KB2013-p120 strain at 1MOI for 24 hours. Next, cells were lyzed using RIPA buffer supplemented with protease inhibitor cocktail and NEM for SLA-DR enrichment using anti-SLA-DR Mab-MY533. Enriched samples were subjected to western blot using ubiquitin antibody and anti-SLA-DRα chain antibody. (D) HEK-293T-SLA-DRα/β stable cells were transfected with plasmids encoding S2, M, N, E and ORF3 for 48 hours. Next, cells were harvested for western blot to evaluate expression of SLA-DRα and β. Cells transfected with empty vector were included as control. Tubulin was probed from the same sample to normalize the total protein load.