Figure 1. Structures of triazolurea (1) and phenylhexanamide (2) mitofusin activators.
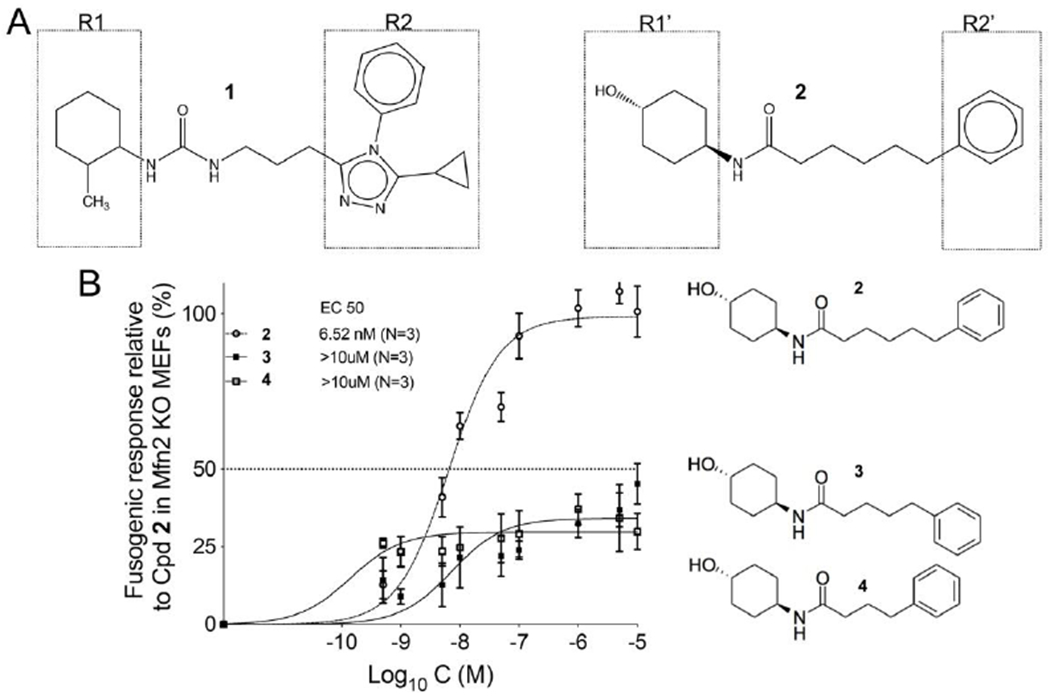
A. Both classes of mitofusin activator share a substituted cyclohexyl (R1, R1’) linked to an aromatic moiety (R2, R2’) by a six or seven member alkyl linker. B. Dose-response relationships for mitochondrial elongation (measured as the increase in mitochondrial aspect ratio) of 2 and its analogs having one (3) or two (4) fewer carbons in the alkyl linker.
