Scheme 2.
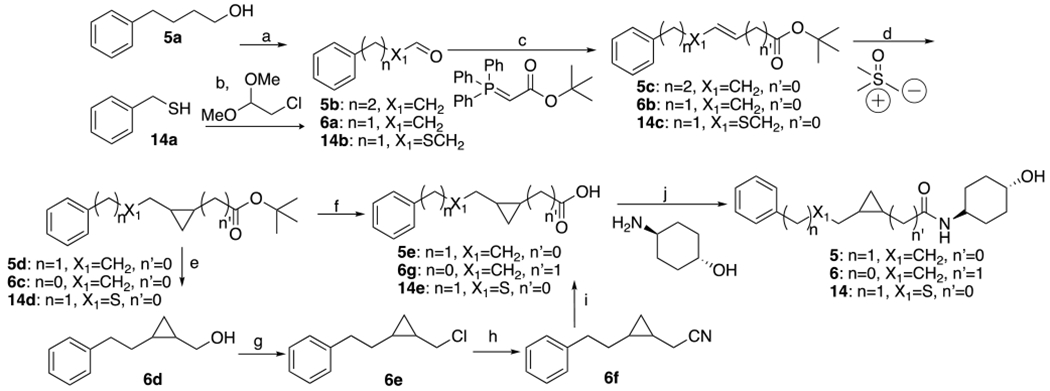
Synthesis of 5, 6 and 14a
aReagents and conditions: (a) oxalyl chloride, DMSO, TEA, DCM, −55-25°C, 20min. (b) i. EtOH, EtONa, KI; ii. 2-chloro-1,1-dimethoxyethane, 80°C, 12h; H2O, H2SO4, 60°C, 12h. (c) THF, 20°C. 5, 14 : 12h, 6: 1h. (d) NaH, DMSO, 20°C, 1.5h. (e) LiAH4, THF, 0-25°C, 3h. (f) TFA, DCM, 25°C, 15h. (g) SOCl2, TEA, CHCl3, 0-70°C, 1h. (h) N(nBu)4CN, THF, 70°C, 12h. (i) KOH, EtOH, H2O, 100°C, 16h. (j) HOBt, EDCI, DIEA, DMV, 25°C. 5, 6: 16h, 14: 2h.
