Figure 6.
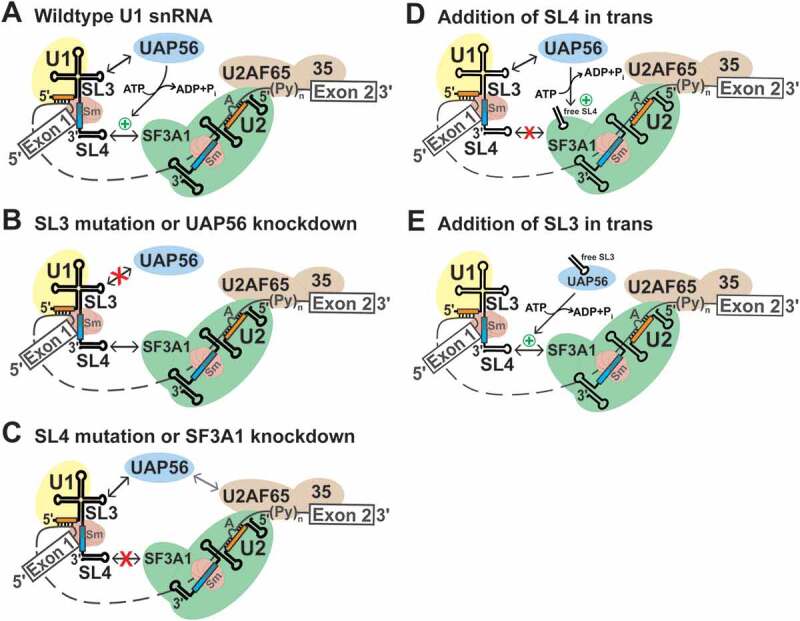
Model for the role of the U1 snRNA during early spliceosome formation. (A) During the early steps of spliceosome assembly, SL3 and SL4 of the U1 snRNA interact with the RNA helicase UAP56 and the U2 snRNP specific protein SF3A1, respectively (double headed black arrows). The SL4-SF3A1 contact bridges the 5′- and 3′-ss complexes. The SL3-UAP56 complex, directly or indirectly, promotes the SL4-SF3A1 interaction (green plus symbol) in an ATP-dependent manner, leading to enhancement of pre-mRNA splicing. (B) Disruption of the SL3-UAP56 contact by either SL3 mutations or UAP56 knockdown prevents stabilization of the SL4-SF3A1 interaction, resulting in reduced splicing. (C) Disruption of the SL4-SF3A1 interaction by either SL4 mutations or SF3A1 knockdown reduces but does not completely abrogate splicing as the SL3-UAP56 interaction can occur. In the absence of the SL4-SF3A1 contact, interaction of UAP56 with U2AF65 likely bridges the 5′- and 3′-ss complexes (grey double headed arrow) [6,55,56]. (D) The addition of excess U1-SL4 in trans competes out the interaction of SF3A1 with endogenous U1 snRNA, reducing A complex formation and inhibiting splicing in vitro [28]. (E) By contrast, addition of excess U1-SL3 in trans enhances pre-mRNA splicing by binding to endogenous UAP56. The U1-SL3-UAP56 complex promotes the SL4-SF3A1 interaction in an ATP-dependent manner, enhancing A complex formation and splicing
