Figure 6.
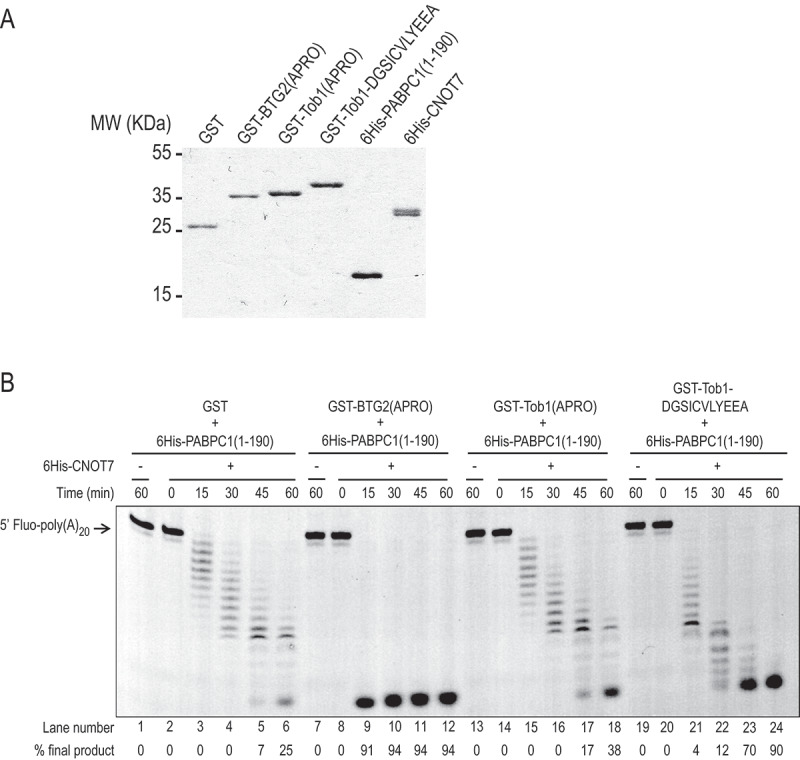
A chimeric Tob1 APRO domain with a boxC motif stimulates CNOT7 deadenylase activity in vitro
A) SDS–PAGE analysis of purified recombinant proteins used in in vitro deadenylation assays. One microgram of purified proteins was resolved on 12% SDS–PAGE gel stained with Coomassie blue. 6His-CNOT7 appeared as a doublet. B) In vitro deadenylation assay with purified CNOT7, wild-type and mutant BTG2 and Tob1 APRO domain derivatives, and first RRM domains of PABPC1. A 5′ Fluorescein-labelled poly(A) substrate of 20 residues was incubated in defined conditions (see Methods) with purified 6His-CNOT7 and 6His-PABPC1(1–190) as well as GST alone, GST-BTG2(APRO) or GST-Tob1(APRO) or GST-Tob1-DGSICVLYEEA as indicated. Reaction products were fractionated on 15% denaturing polyacrylamide gel. Experiments were performed three times with similar results.
