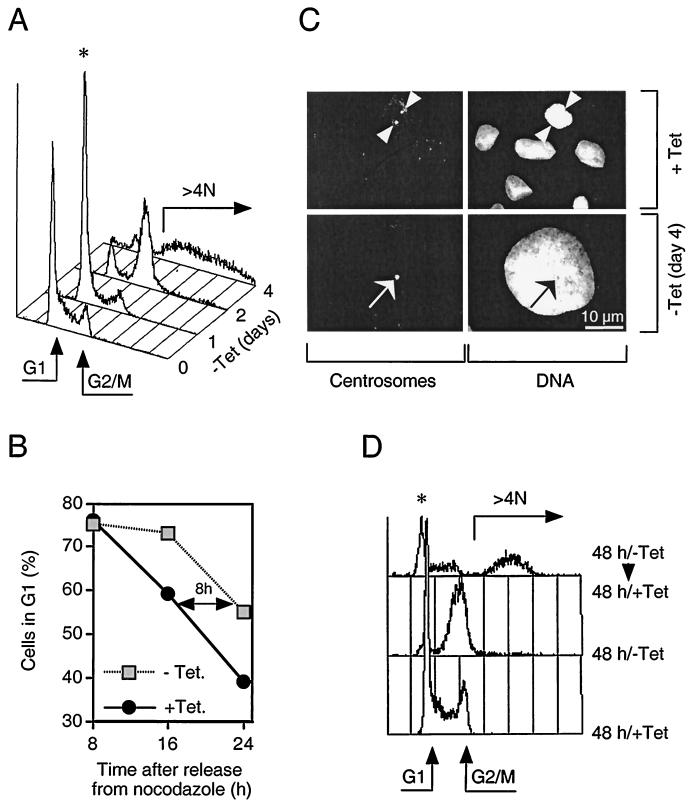
FIG. 2.
Induction of myc-Cdh1 prevents cell division and promotes endoreplication. (A) U-2-OS Cdh1 cells were stimulated to express myc-Cdh1 by removal of tetracycline (−Tet). At the indicated time points after myc-Cdh1 induction, the cells were fixed, stained with propidium iodide, and analyzed for DNA content by single-parameter fluorescence-activated cell sorting. Arrows indicate the position of G1 and G2/M cells as well as the degree of endoreplication (over 4 N DNA content); the asterisk indicates the initial transient accumulation of cells in G1 phase. (B) U-2-OS Cdh1 cells were synchronized in metaphase by incubating in the presence of nocodazole. Rounded mitotic cells were collected and replated into a drug-free medium. At the same time, expression of myc-Cdh1 was induced by removing tetracycline from the medium (−Tet). At indicated time points, the cells were fixed, and their cell cycle distribution was determined by flow cytometry. (C) U-2-OS Cdh1 cells were seeded on glass coverslips and either cultured in the constant presence of tetracycline (+Tet) or induced to express myc-Cdh1 for 4 days (−Tet day 4). The cells were immunostained with an antibody against pericentriolar proteins (left panels). DNA was counterstained with Hoechst 33258 (right panels). The representative images at both time points show identical fields for centrosome and DNA staining, respectively (arrowheads, separated centrosomes; arrows, unseparated centrosomes). (D) U-2-OS Cdh1 cells were either grown in the constant presence of tetracycline (+Tet) or induced to express myc-Cdh1 by removal of tetracycline for 48 h (−Tet). When indicated, the expression of ectopic Cdh1 was silenced by readdition of tetracycline into the culture media, and the cells were incubated for an additional 48 h (−Tet/+Tet). The DNA content was analyzed by FACS.