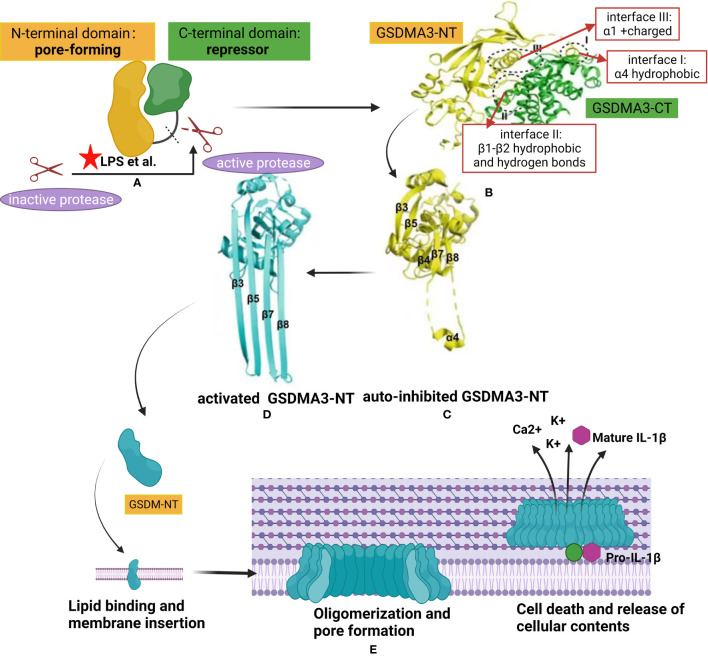
Figure 2.
Structural auto-inhibition in GSDM family and mechanism of gasdermin membrane insertion and pore formation. (A) Interdomain interaction between the N-terminal of gasdermin (GSDM-NT) and C- terminal of gasdermin (GSDM-CT), with pore-forming and auto inhibitory characteristics respectively, keeps the full length GSDM protein in an auto-inhibited state. Different proteases, such as inflammatory caspases or granzymes, are activated by danger signals from pathogens like lipopolysaccharide (LPS). The latter was represented by a red pentacle in the image. The linker region of GSDMs is cleaved by the active protease releasing the GSDM-NT from the GSDM-CT. (B) (48) X-ray crystal structure of full-length GSDM and the interfaces mediating the inter-domain interactions (I-III) by forming electrostatic, hydrophobic, and hydrogen bonding. The GSDM-NT and GSDM-CT domains are colored yellow and green, respectively. (C, D) (49) Crystal structure of GSDMA3-NT in auto-inhibited conformation (C) and the pore conformation (D). (E) A proposed universal model showing the pore formation by GSDM family. Once released from the GSDM-CT, the GSDM-NT is recruited to insert in the cell membrane by binding with lipid. Upon membrane binding, the GSDM-N concentrates and starts the oligomerization process forming pores leading to release of cellular contents,including ion flux and mature IL-1β, and finally cell death.