Figure 2.
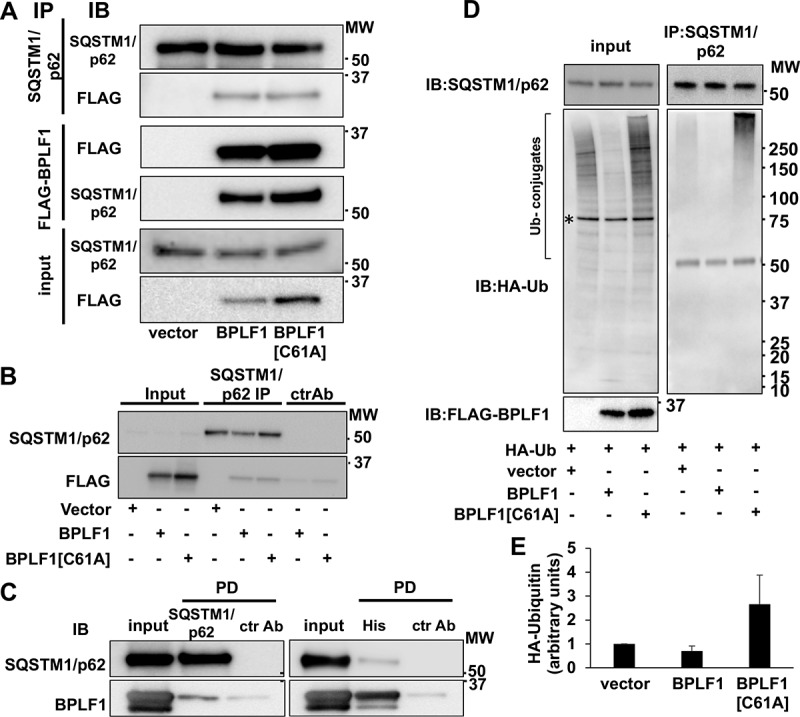
SQSTM1/p62 is a bona fide BPLF1 substrate. (A) Interaction of BPLF1 with the autophagy receptor protein SQSTM1/p62 was validated by reciprocal co-immunoprecipitation (IP). Western blots (WB) from representative experiments are shown. (B) Isotype control of SQSTM1/p62 immunoprecipitation. Weak non-specific binding of BPLF1 to the sepharose beads was observed with isotype control precipitation. (C) In vitro affinity isolation experiment shows direct binding of BPLF1 to SQSTM1/p62. (D) To resolve noncovalent protein interactions, endogenous SQSTM1/p62 was immunoprecipitated in denaturing conditions. Precipitated SQSTM1/p62 was free of ubiquitin in the presence of catalytically active BPLF1 and heavily ubiquitinated in the presence of the catalytic mutant. A non-specific 75 kDa band detected in total cell lysates by the anti-HA antibody is indicated by an asterisk. The antibody heavy chain is detected as a 50 kDa band in the IP blot. (E) Densitometry quantification of the ubiquitin smear in two independent experiments
