Figure 4.
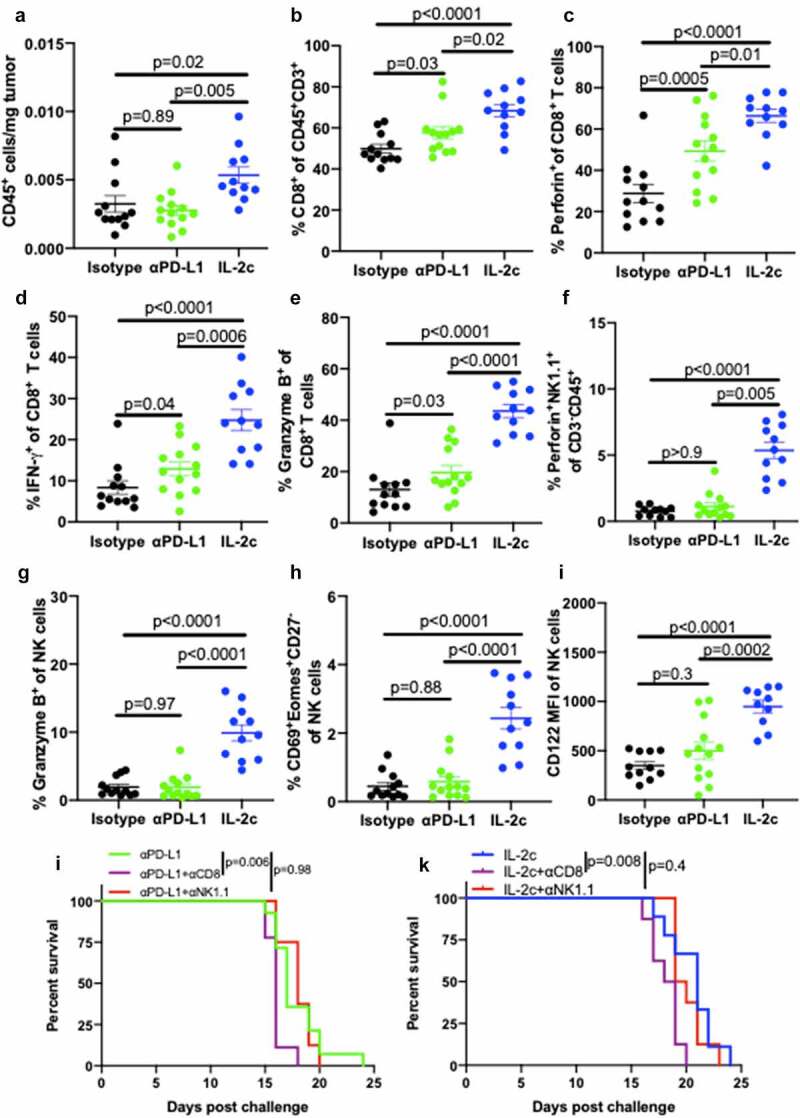
IL-2c activates of CD8+ T cells and promotes NK cell maturation in peritoneal B16. Wild-type male mice were challenged peritoneally with 4 × 105 B16 cells and treated with 100 μg αPD-L1 on days 7, 12, 17 or IL-2c on days 7, 9, 11, 13. Mice were sacrificed on day 15 for flow cytometric analysis of immune (CD45+) cell number normalized to tumor weight (a). (b-e) Intratumoral CD8+ T cell frequency (b) and effector molecule production (c-e). (f-i) Intratumoral NK cell perforin (f) and granzyme B (g) production, CD69+ Eomes+ expression by CD27− NK cells (h), and CD122 expression (i). N = 11–13 mice/group. p value, one-way ANOVA. (j) Survival of wild-type male mice challenged peritoneally with 4 × 105 B16 cells and treated with αPD-L1 (as above) ± 250 ug αNK1.1 or 250 ug αCD8 on days 6, 9, 12, 15, 18. N = 8–14 mice/group. p value, log-rank. (k) Survival of wild-type male mice challenged peritoneally with 4 × 105 B16 cells and treated with IL-2c (as above) ± 250 ug αNK1.1 or 250 ug αCD8 on days 6, 9, 12, 15. N = 8–9 mice/group. p value, log-rank. Note: Survival of mice in Figure 4j,k can be compared to survival of isotype-treated mice with peritoneal B16 in Fig. S7B. ANOVA, analysis of variance. Eomes, Eomesodermin. IFN-γ, interferon-gamma. IL-2, interleukin-2 complex. MFI, Mean Fluorescence Intensity. NK, Natural Killer. PD-L1, Programmed Death Ligand 1
