Figure 5.
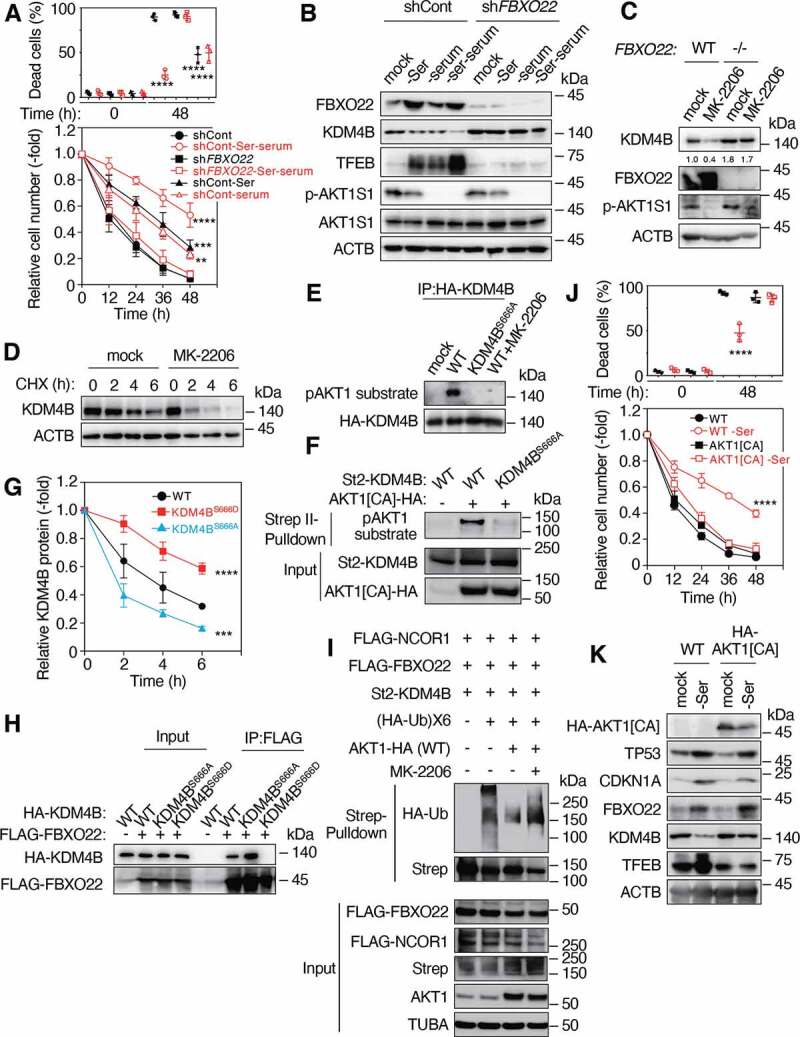
Mitogen-activated AKT1 phosphorylates KDM4B and inhibits its FBXO22-mediated degradation. (A) RPE-1 cells expressing the indicated Dox-inducible shControl or shFBXO22, preconditioned with or without serine depletion plus serum starvation, followed by incubation with low glucose (2 mM) media for 48 h. The proportions of dead cells (upper panel) and the relative cell numbers (lower panel) were determined at the indicated times as in Figure 1A. (B) Lysates from cells as in (A) were subjected to immunoblotting using the indicated antibodies. (C) WT or FBXO22−/− HeLa cells were treated with or without MK-2206 for 4 h and the lysates were subjected to immunoblotting using the indicated antibodies. (D) FBXO22−/− HeLa cells were treated with or without 20 μM MK-2206 together with 50 μg/mL CHX. The lysates were collected at the indicated times and subjected to immunoblotting using the indicated antibodies. (E) Lysates from 293 T cells expressing HA-KDM4B WT or KDM4BS666A treated with or without 20 μM MK-2206 were immunoprecipitated using anti-HA affinity gel. The resultant immunoprecipitates were subjected to immunoblotting. (F) 293 T cells were transfected with or without WT St2-KDM4B or its KDM4BS666A mutant and/or AKT1[CA] lysed under denaturing conditions and subjected to StrepTactin pulldown, followed by immunoblotting. (G) HeLa cells expressing the WT KDM4B or its mutants were treated with 50 μg/mL CHX. The lysates were collected at the indicated times and subjected to immunoblotting, and the relative KDM4B intensities were determined using ImageJ. (H) Lysates from HeLa cells expressing the indicated genes were immunoprecipitated using anti-FLAG M2 affinity gel. The resultant immunoprecipitates were subjected to immunoblotting. (I) FBXO22−/− HeLa cells were transfected with the indicated genes, treated with MG132, lysed under denaturing conditions and subjected to StrepTactin pulldown, followed by immunoblotting. (J) WT RPE-1 cells or cells expressing AKT1[CA], preconditioned with or without serine depletion and then treated with low glucose (2 mM) media for 48 h. The proportions of dead cells (upper panel) and the relative cell numbers (lower panel) were determined at the indicated times. (K) Lysates from cells preconditioned as in (J) were subjected to immunoblotting using the indicated antibodies. Data are presented as means±s.d. of three independent experiments. One-way ANOVA with Dunnett’s multiple comparisons post hoc test was performed against a control (shCont or WT) group mean (A, G, J). **P < 0.01, ***P < 0.001, ****P < 0.0001
