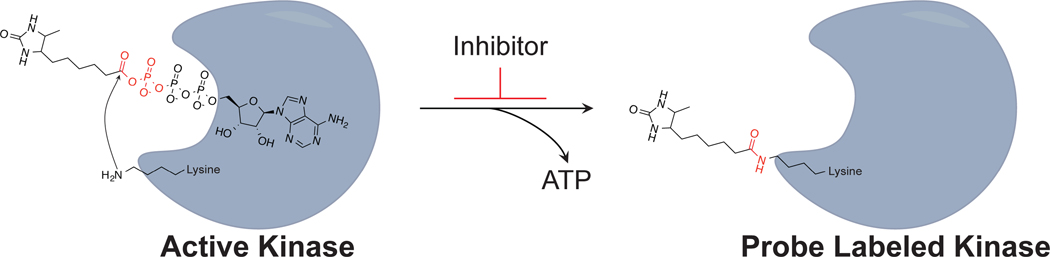
Figure 1. Mechanism of ATP acyl phosphate probe binding.
The probe ATP moiety enters the active kinase ATP-binding domain and where the acyl phosphate group is positioned in proximity to a conserved lysine residue. The lysine residue reacts with the probe to release ATP, which results in covalent modification of the kinase with a desthiobiotin reporter-tag to permit further subsequent analyses. Pre-treatment of cell lysates with inhibitor to inactivate proteins results in blockade of probe labeling.