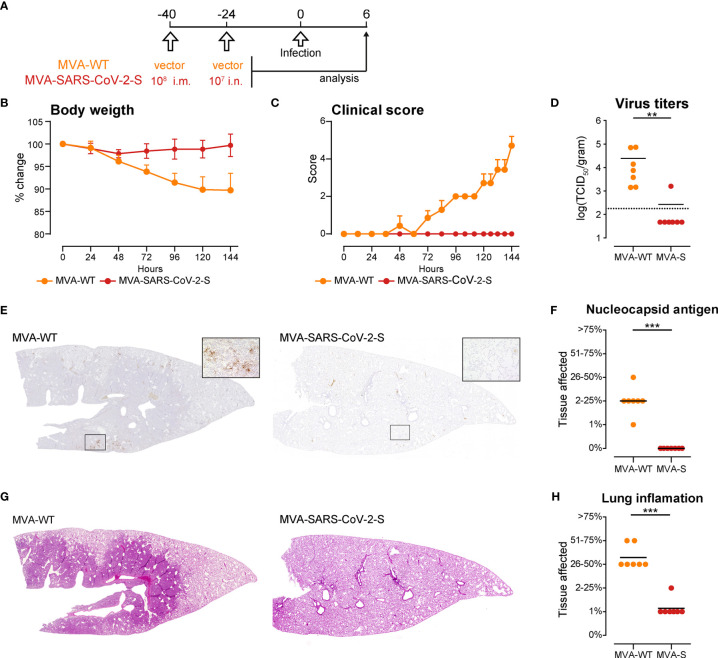
Figure 6.
Intranasal immunization with MVA-SARS-2-S protects hamsters from SARS-CoV-2 infection. (A) Immunization and infection protocol scheme. (B, C) Relative body weight (B) and clinical scores (C) of hamsters after infection with SARS-CoV-2. (D) SARS-CoV-2 virus titers in the lungs measured by quantitative RT-PCR. (E) Representative stitched photomicrographs showing an overview of the entire left lung lobe stained for SARS-CoV-2 nuceloprotein (brown staining). Original magnification 40x, inset 400x. (F) Semiquantitative scoring of SARS-CoV-2 nuceloprotein per lung section averaged on 2 lung sections per hamster. (G) Representative stitched photomicrographs showing an overview of the entire left lung lobe stained with hematoxylin and eosin (H, E) indicate severe inflammation in the lungs of hamsters immunized with control, MVA-WT virus, which is almost not existent in the lungs of hamsters immunized with MVA-SARS-CoV-2. Original magnification 40x. (H) Lung inflammation scores calculated as described supplemental methods. (B–H) Pooled data from one experiment with n = 7 per group. Individual values (signs) and mean group value (line). Statistical analysis was done using Mann-Whitney t test. **p < 0.01, ***p < 0.001.