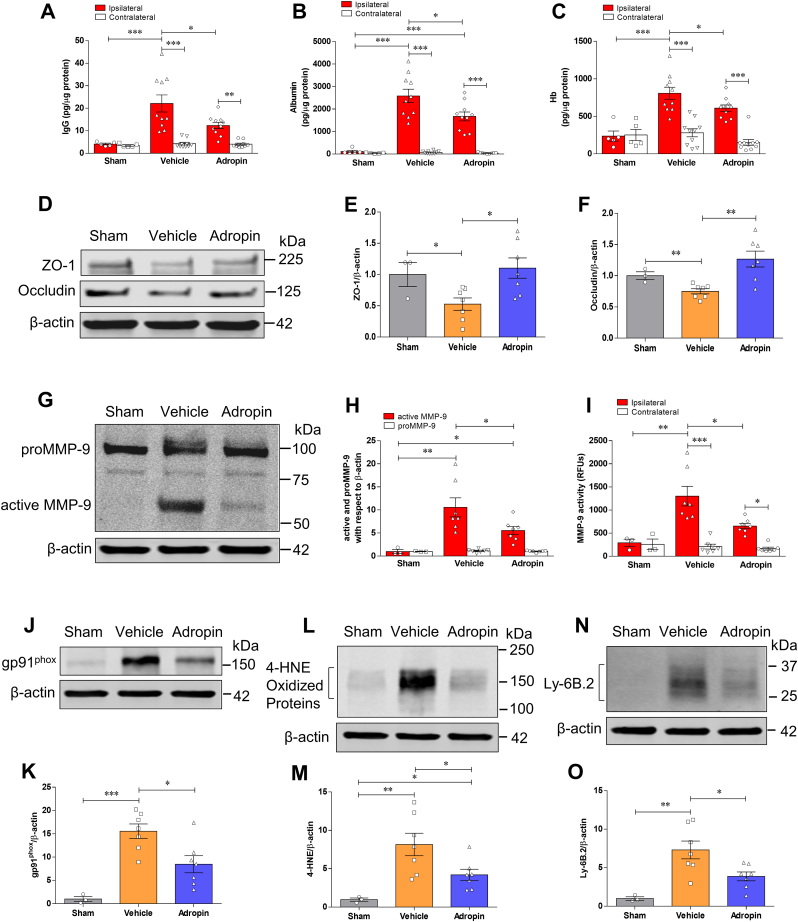
Fig. 4.
Treatment with synthetic adropin peptide reduces neurovascular injury, MMP-9 activity, oxidative damage, and neutrophil infiltration in the ischemic brain. Adult (10–12 weeks) male mice received one bolus injection of vehicle or synthetic adropin34-76 peptide (900 nmol/kg; i.v.) at the onset of ischemia and were euthanized 24h after pMCAO and cerebral cortex from ipsilateral and contralateral sides were collected for molecular analysis. Sham-operated mice received the same surgical procedures except for the MCA occlusion. A-C, Stroke induced a dramatic BBB damage, which was assessed by increased levels of IgG and albumin as well as hemorrhagic transformation quantified by increased hemoglobin level in the ipsilateral cortex, and post-ischemic treatment with adropin significantly reduced the increased levels of these markers. Two-way ANOVA with Bonferroni post-tests, *P < 0.05, **P < 0.01, ***P < 0.001. Sham (n = 5), Vehicle (n = 10), Adropin (n = 10). D, Representative immunoblots for tight junction proteins ZO-1 and occludin in homogenates from the ischemic cortex. β-actin was used as a loading control. E, F, Densitometric analysis shows that stroke resulted in significant degradation of ZO-1 and occludin in the ischemic cortex. The degradation of these two tight junction proteins was dramatically attenuated in animals receiving adropin treatment. One-way ANOVA with Bonferroni post-tests, *P < 0.05, **P < 0.01. Sham (n = 3), Vehicle (n = 7), Adropin (n = 7). G-I, Treatment with adropin significantly reduced MMP-9 levels measured by Western blot (G, H) and enzymatic activity by immunocapture assay (I) in the ipsilateral cerebral cortex compared to the vehicle group. RFUs: relative fluorescence units. One-way ANOVA with Bonferroni post-tests, *P < 0.05, **P < 0.01, ***P < 0.001. Sham (n = 3), Vehicle (n = 7), Adropin (n = 7). J-O, Representative Western blots, and densitometric analysis showed that stroke induced a dramatic increase in oxidative stress markers, NADPH oxidase isoform NOX2 (gp91phox) and 4-hydroxy-2-nonenal (4-HNE)-modified proteins, as well as infiltration of neutrophils (Ly-6B.2 as a specific neutrophil marker) into the ischemic cerebral cortex. Treatment with adropin significantly reduced the increased levels of these oxidative stress and inflammatory markers. One-way ANOVA with Bonferroni post-tests, *P < 0.05, **P < 0.01, ***P < 0.001. Sham (n = 3), Vehicle (n = 7), Adropin (n = 7).