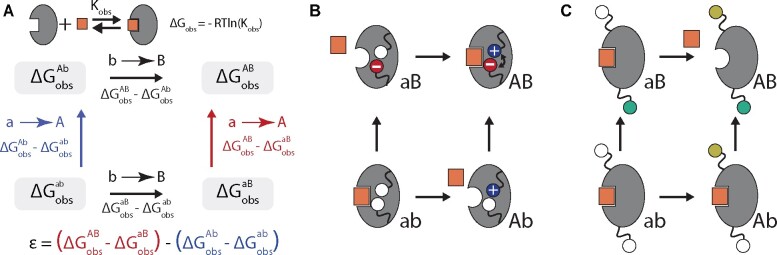
Figure 1.
Mechanistic and mathematical descriptions of epistasis. (A) The mathematical description of epistasis (ε) in ligand binding free energy () for the mutant cycle between genotypes ab and AB. measures the strength of the binding interaction between protein (gray) and ligand (orange). We indicate genotypes as superscripts. ε is defined as the difference in the effect of mutation in the aB background (red text), vs its effect in the ab background (blue text). (B) Mutant cycle where epistasis is readily understood: the and mutations introduce charges into the hydrophobic core, destabilizing the protein and disrupting binding of the orange square. Mutations and lead to a new electrostatic interaction when introduced together (minus and plus signs) restoring stability and binding. (C) Mutant cycle with difficult-to-understand epistasis. Mutations at two distant sites (green and yellow spheres) have no effect on binding of the orange square when introduced independently, but disrupt binding when introduced together.