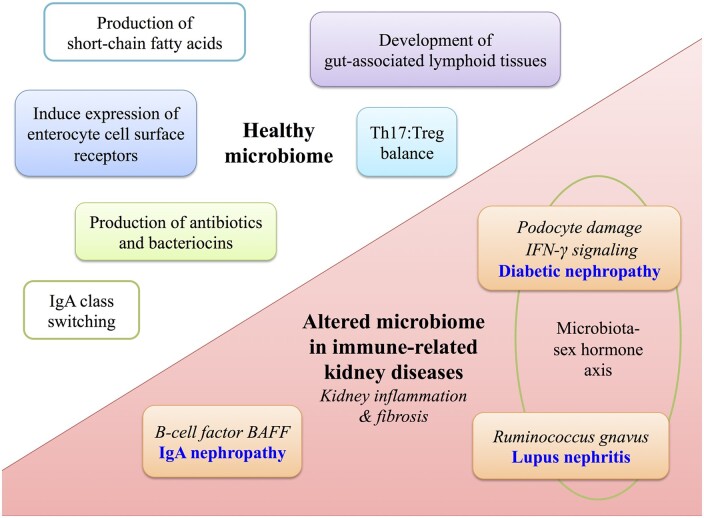
FIGURE 1.
Role of the gut microbiome in health and in autoimmune kidney diseases. Upper left: the healthy gut microbiome shapes local and systemic immunity including production of antibiotics and bacteriocins, induction of surface pattern recognition receptors on host intestinal cells and production of SCFAs. SCFAs provide a nutrient source to host intestinal cells and also modulate immune function via induction of natural killer cells and expansion of Tregs. The microbiota also modulates development of gut-associated lymphoid tissues (Peyer’s patches, mesenteric lymph nodes), shapes the balance between cytotoxic Th17 and Treg cells and induces IgA class switching. Lower right: the microbiome is altered in CKD and bacterial-derived uremic toxins translocate across the leaky gut and induce systemic effects including kidney inflammation and fibrosis. In diabetic nephropathy, the toxin phenyl sulfate induces podocyte damage and proteinuria. There is a microbiota–sex hormone axis whereby transfer of microbiota from male to female mice increases IFN-γ signaling and reduced incidence of diabetes. In lupus nephritis, higher gut abundance of Ruminococcus gnavus strain 2 is associated with more severe lupus nephritis kidney pathology. A microbiota–sex hormone axis is also observed, whereby female mice have more severe disease. Mouse studies indicate that overexpression of BAFF, a B-cell factor crucial for IgA synthesis, develop a commensal flora-dependent IgA nephropathy.