Summary
The abscisic acid (ABA) signalling pathway is involved in the plant response to osmotic stress caused by drought and/or salinity. Although the ABA signalling pathway has been elucidated in Arabidopsis, it remains elusive in woody poplars. In this study, genome‐wide analyses of U‐box genes in poplars revealed that a U‐box E3 ubiquitin ligase gene, PalPUB79, is significantly induced following drought, salinity and ABA signalling. PalPUB79 overexpression enhanced drought tolerance in transgenic poplars, while PalPUB79 RNAi lines were more sensitive to drought. PalPUB79 positively regulated ABA signalling pathway. Furthermore, PalPUB79 interacted with PalWRKY77, a negative transcriptional regulator of ABA signalling, and mediated its ubiquitination for degradation, therefore counteracting its inhibitory effect on PalRD26 transcription. However, the finding that PalWRKY77 negatively regulates PalPUB79 expression was indicative of a negative feedback loop between PalWRKY77 and PalPUB79 during ABA signalling in poplar. These findings provide novel insight into the mechanism through which PalPUB79 enhances the ABA‐mediated stress response in woody poplars.
Keywords: PalPUB79, U‐box type E3 ubiquitin ligases, drought, abscisic acid, Populus
Introduction
Drought, one of the most important adverse environmental events, causes water deficit among plants and can disproportionately affect plant growth (Barber et al., 2000; Hu and Xiong, 2014; Tardieu et al., 2018). Drought stress can occur at any stage of plant growth and, as such, may seriously affect plant survival, development, flowering, fruit set and total productivity depending on the intensity and duration of the event (Boyer, 1982; Venuprasad et al., 2007). A prominent feature of the plant response to drought stress is the rapid accumulation of the phytohormone abscisic acid (ABA), which induces stomatal closure to decrease transpirational water loss (Zhu, 2002). ABA is mainly biosynthesized in the cytoplasm and plastids (Nambara and Marion‐Poll, 2005), and a series of key enzymes are responsible for consequential steps of ABA biosynthesis (Cheng et al., 2002; North et al., 2010; Schwartz et al., 2003; Seo et al., 2010; Xiong et al., 2001; Xiong et al., 2002). ABA is recognized by the ABA receptor and multiple receptors identified as PYRABACTIN RESISTANCE1 (PYR1)/PYR1‐LIKE (PYL)/REGULATORY COMPONENTS OF ABA RECEPTORS (RCAR) proteins. These receptors, upon activation by ABA, can interact with the type 2C protein phosphatases (PP2Cs) to reverse the inhibitory effects of PP2Cs on downstream signals (Zhu, 2016), such as the SUCROSE NONFERMENTING 1‐RELATED PROTEIN KINASE 2 (SnRK2) protein‐mediated phosphorylation of the ABI5 and ABFs transcription factors (Boudsocq et al., 2004; Furihata et al., 2006).
Plants subjected to drought stress or ABA treatment will show transcriptional changes in numerous genes (Dong et al., 2015); this indicates just how critical transcriptional regulation is for ABA‐dependent stress responses. Besides ABI5 and ABFs, genes from the ERF, MYB, NAC and WRKY families are also involved in the ABA signalling cascade that underlies the plant response to drought stress (Hu and Xiong, 2014; Rohit et al., 2016). For instance, the overexpression of RESPONSIVE TO DESICCATION 26 (RD26), which encodes a NAC transcription factor, in Arabidopsis increases plant tolerance to drought stress (He et al., 2019). In Populus, ABSCISIC ACID RESPONSE ELEMENT (ABRE) motifs in the promoters of drought‐responsive genes PtrNAC006, PtrNAC007 and PtrNAC120 are activated by trimeric AREB1‐ADA2b‐GCN5 protein complexes during drought stress (Li et al., 2018). There is empirical evidence that the WRKY gene family plays an important role in the plant stress signalling pathway (Liu et al., 2019). In Arabidopsis, high ABA levels can relieve AtWRKY40‐mediated repression of the ABA‐responsive genes, i.e., ABI4 and ABI5, by translocating AtWRKY40 from the nucleus to the chloroplast envelope (Shang et al., 2010). In our previous study on woody poplars, PalWRKY77 negatively regulated responses to salt stress and ABA signalling by directly binding to the W‐boxes of the promoters of PalNAC002 and PalRD26 to inhibit their expression (Jiang et al., 2020). Although enhanced ABA levels have been shown to repress PalWRKY77 transcription, the mechanism through which ABA signalling removes the negative effect of PalWRKY77 in woody poplars remains unclear.
The ubiquitin‐proteasome system (UPS) is involved in the plant response to drought, salinity and ABA signalling (Kelley, 2018; Xu and Xue, 2019). The UPS is composed of target proteins, the 26S proteasome, ubiquitin (Ub), Ub‐activating enzyme (E1), Ub‐conjugating enzyme (E2) and ubiquitin ligase (E3) (Xu and Xue, 2019). Among these, ubiquitin ligase plays a critical role in determining the specificity of the ubiquitination system by screening candidate proteins (Ciechanover, 1998). The E3 ubiquitin ligase family can be divided into RING (REALLY INTERESTING NEW GENE), HECT (HOMOLOGOUS TO THE E6AP CARBOXYL TERMINUS), Kelch and plant U‐box (PUB) members (Hua and Vierstra, 2011). A recent study found PUB family members to be involved in the ABA pathway and drought stress response. More specifically, Arabidopsis PUB12 and PUB13 interact with ABI1 to regulate the response to ABA (Kong et al., 2015). The double mutant pub18 pub19 Arabidopsis displayed enhanced stomatal closure when exposed to ABA, with these plants more tolerant to drought stress (Seo et al., 2016). This result can be explained by PUB18‐ and PUB19‐mediated degradation of Exo70B1, which was found to respond to ABA signalling through knock‐out mutant experiments (Seo et al., 2016). Moreover, heterologous overexpression of the soybean PUB8 in Arabidopsis inhibited ABA‐mediated stomatal closure, which resulted in plants that were more sensitive to drought (Wang et al., 2016). However, only a few studies have focused on the relationship between PUBs and the ABA pathway in perennial trees.
In this study, we investigated the expression of the 91 U‐box E3 ubiquitin ligase genes of Populus alba var. pyramidalis during salt stress, mannitol treatment, and ABA signalling. We identified one differentially expressed gene, termed PalPUB79, during these experiments, and isolated it through RNA sequencing and quantitative real‐time PCR (qRT‐PCR). Phylogenetic analyses indicated that PalPUB79 and AtPUB19 derive from different ancestors, which suggests functional diversity. Overexpression of PalPUB79 enhanced drought tolerance among transgenic poplars, and this phenotype was eliminated in the absence of ABA signalling. In addition, PalPUB79 RNA interference (RNAi) poplars showed compromised drought tolerance. These results indicate that PalPUB79 positively regulates drought response in an ABA‐dependent manner. PalPUB79 interacted with PalWRKY77 to mediate the ubiquitination and degradation of PalWRKY77, eventually easing PalWRKY77‐mediated inhibition of ABA‐responsive genes. In addition, we found that PalWRKY77 can repress PalPUB79 expression by binding to the W‐box in its promoter. These results suggest that PalPUB79 is an ABA‐dependent positive regulator of the drought response signalling pathway in poplars.
Results
Identification of U‐box E3 ubiquitin ligases in poplars
The genomic sequencing of Populus alba var. pyramidalis is a valuable starting point for molecular functional studies in this species (Ma et al., 2019). An HMM search program, i.e., a Hidden Markov Model of the U‐box domain (PF04564; http://pfam.xfam.org/) with a cut‐off E‐value of 0.1, was run to identify U‐box containing genes in the Populus alba var. pyramidalis genome. The results were subsequently manually inspected. A total of 91 genes harbouring the U‐box domain were identified in P. alba var. pyramidalis (Table S1). Using the same method, 90 members of the same U‐box family were also identified in the Populus trichocarpa genome (Table S2). A phylogenetic tree was constructed based on the putative full‐length peptide sequences of the 91 proteins from P. alba var. pyramidalis, 90 members from P. trichocarpa and 64 members from Arabidopsis (Figure S1). According to the topology of the phylogenetic tree and the taxonomy of U‐box members in Arabidopsis (Mudgil et al., 2004), all of the poplar U‐box proteins could be divided into several clustered groups: KINASES; ARMADILLO/BETA‐CATENIN‐LIKE (ARM) REPEAT; and U‐BOX N‐TERMINAL DOMAIN (UND)/ARM REPEAT (Figure S1).
PalPUB79 is induced by salt, mannitol and ABA treatments
We analysed RNA sequencing data collected from plants under salt treatment to determine the expression patterns of poplar U‐box genes in response to abiotic stress. The gene ontology (GO) enrichment analysis revealed that many of the genes with increased expression were involved in water deprivation, stomatal movement, the ABA signalling pathway and hyperosmotic response (Figure 1a), while the down‐regulated genes were related to cell growth, growth‐related phytohormone signalling, along with DNA and RNA biosynthesis (Figure 1b). These results indicate that salt stress can induce osmotic stress and inhibit growth processes in poplars. Among the 478 differentially expressed genes (P < 0.05) (Table S3), 376 genes showed enhanced expression levels after exposure to salt (Figures 1c, d). Interestingly, only two U‐box genes (PalPUB79 and PalPUB88) were found to be up‐regulated by salt treatment, with PalPUB79 induced more significantly than PalPUB88 (Table S3). On the other hand, only one U‐box gene (RAD5B) was down‐regulated after salt treatment (Table S3). We also analysed the expression profile of PalPUB79 after salt, mannitol and ABA treatment by quantitative PCR (qPCR). PalPUB79 was rapidly induced after each of these three treatments, reaching an expression peak at 1 h and then slowly decreasing in the following hours (Figure 1e). Promoter analysis also revealed that certain cis‐elements are involved in drought stress and the ABA response, e.g., ABRE and MBS in the promoter of PalPUB79 (Figure S2).
Figure 1.
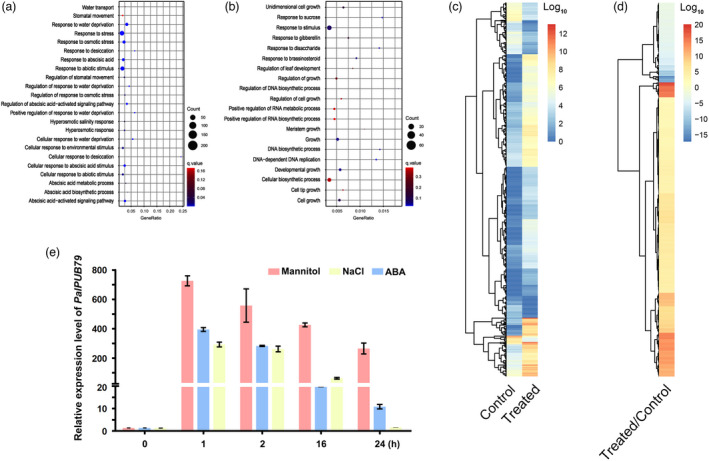
The transcriptome of Populus alba var. pyramidalis under salt stress, along with the expression pattern of PalPUB79 under mannitol and ABA treatments. (a) The gene ontology (GO) enrichment analysis of the genes with up‐regulated and (b) with down‐regulated expression under salt treatment. All genes analysed based on GO enrichment show differential expression at a significance level of P < 0.05. (c–d) Heatmaps of the differential expression (P < 0.05) of 478 genes with or without salt treatment. (e) The expression pattern of PalPUB79 under mannitol, salt and ABA treatments.
Characterization of PalPUB79
An analysis of PalPUB79 primary structure revealed that the N‐terminal UND domain was followed by a U‐box domain, with five ARM domains at the C‐terminus (Figure S3a). Further phylogenetic analyses of all the PUBs in P. alba var. pyramidalis, P. trichocarpa and Arabidopsis indicated that PalPUB79 has the most sequence similarity to AtPUB19 among all of the reported PUB homologues in Arabidopsis. However, a comparison of the PalPUB79 and AtPUB19 sequences demonstrated that these two proteins differ from each other, with only 41.15% sequence identity (Figure S3b). To further determine the evolutionary relationship between these two genes, we used all of the sequences homologous to PalPUB79 and AtPUB19 in various plant species (including basal angiosperms) to construct a phylogenetic tree (Figure 2a). Based on the results, it became obvious that these two genes are paralogous, i.e., originated from independent gene duplications from different ancestors in the evolutionary diversification of angiosperm plants, rather than orthologous. These duplications and divergences may have led to functional differences among the homologous genes from different species. The phylogenetic tree showed significant divergence for PalPUB79 and AtPUB19, and both had multiple closely related homologues that could be explained through recent independent whole‐genome duplications (WGDs) in their respective direct ancestor nodes. All of the PalPUB79 orthologues in Populus and Salix belonged to one monophyletic subclade, sister to another subclade comprising another set of paralogues from poplars and willows. These two subclades originated from the recent Salicaceae‐specific WGD. Therefore, we renamed the gene described in this study as PalPUB79 rather than PalPUB19, which was the previous decision according to the remotely related paralogue AtPUB19 (Jiang et al., 2020).
Figure 2.
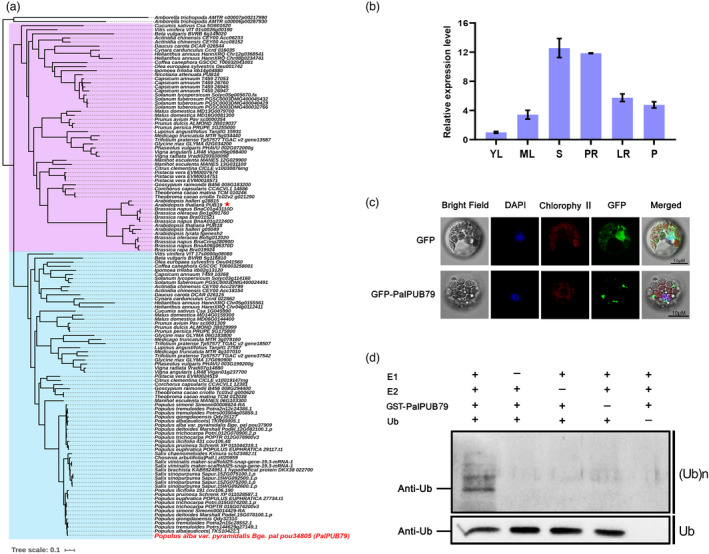
Characteristics of PalPUB79. (a) Phylogenetic tress constructed from the full‐length peptide sequences of PalPUB79 and AtPUB19 homologues from multiple plants, including basal angiosperms. The PalPUB79 is highlighted in red while AtPUB19 is denoted by red star. (b) The tissue expression profile of PalPUB79 in Populus alba var. pyramidalis, based on relative PalPUB79 levels, in young leaf (YL), mature leaf (ML) stem (S), primary root (PR), lateral root (LR) and petiole (P). Error bars indicate SD (n = 3). (c) The subcellular localization of PalPUB79 in P. alba var. pyramidalis protoplasts. The pBI221 empty vector served as a control, while 4′,6‐diamidino‐2‐phenylindole (DAPI) staining was used to identify the nucleus. (d) The E3 ubiquitin (Ub) ligase activity of PalPUB79 was detected using the GST‐PalPUB79 fusion protein, His‐Ub UBCH5C (E2) and E1 (R&D Systems, E‐300‐050). An anti‐Ub antibody was used to detect the ubiquitinated proteins (top panel) and ubiquitin monomers (bottom panel).
The tissue expression profile of PalPUB79 was further analysed by qPCR. The results showed that PalPUB19 is expressed across all tissues; moreover, it was dominantly and significantly expressed in stems and primary roots, followed by lateral roots and petioles (Figure 2b). The subcellular localization analysis of the PalPUB79‐GFP fusion protein revealed a punctate pattern in the cytoplasm; in contrast, free GFP was detected in the cytomatrix and nucleus (Figure 2c). The purified MBP‐PalPUB79 possessed E3 ubiquitin ligase activities that were detected using anti‐Ub antibodies; this supports that PalPUB79 encodes an E3 ubiquitin ligase (Figure 2d).
PalPUB79 confers drought tolerance in transgenic poplars
To identify the function of PalPUB79, two transgenic lines (PalPUB79‐OE‐L14 and PalPUB79‐OE‐L18) with high expression levels of PalPUB79 and two RNAi lines (PalPUB79‐Ri‐L2 and PalPUB79‐Ri‐L12) were obtained and selected for further analysis (Figures S4 and S5). Neither the overexpression nor RNAi lines showed any significant differences in plant development or growth under normal conditions when compared to wild‐type (WT) plants (Figures 3a, b and S6, S7). We subjected the 45‐day‐old overexpression, RNAi and WT poplar plantlets to drought conditions in the greenhouse for 5 days, after which the plants were rehydrated for 2 days. Wilting and drying occurred across all plantlets, but the RNAi lines demonstrated the most severe reaction, while the lines with high PalPUB79 expression showed the best tolerance to drought conditions (Figure 3a, b). After 2 days of rehydration, the lines with high PalPUB79 expression regained vitality, while the RNAi lines experienced serious necrosis relative to WT plants (Figure 3a, b). In addition to marked phenotypic differences, PalPUB79‐OE‐L14 and PalPUB79‐OE‐L18 plantlets displayed lower MDA content and electrolyte leakage than WT plants, yet higher photosynthetic pigment content relative to WT plants (Figure 3c–e). On the other hand, the PalPUB79‐Ri‐L2 and PalPUB79‐Ri‐L12 plantlets showed higher MDA content and electrolyte leakage than WT plants, but lower photosynthetic pigment content relative to WT plants (Figure 3f–h).
Figure 3.
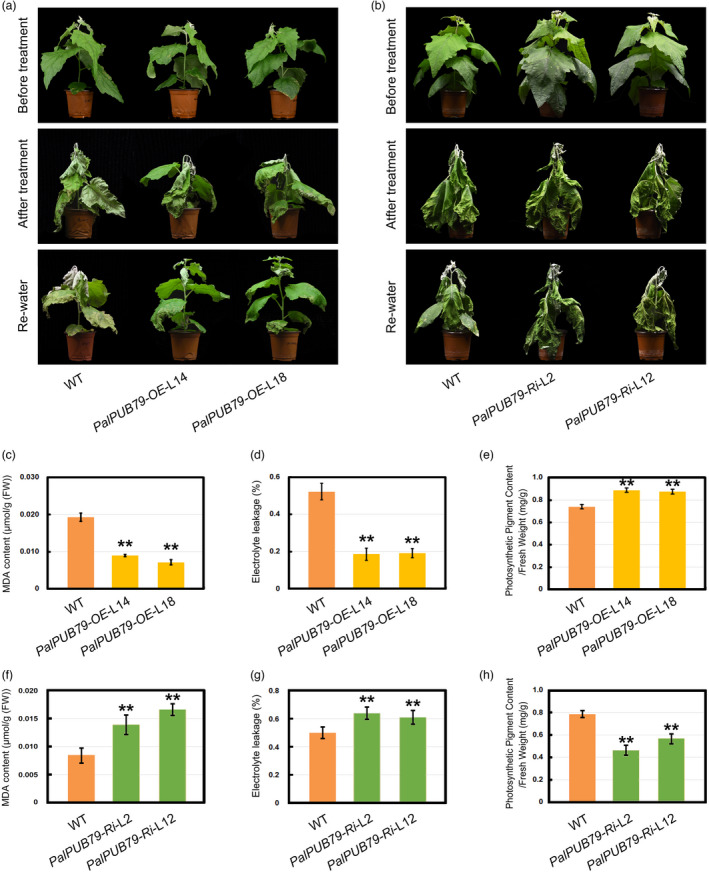
Drought tolerance among PalPUB79 overexpression and RNAi poplars. (a) The transgenic Populus tomentosa overexpressing PalPUB79 (PalPUB79‐OE‐L14 and PalPUB79‐OE‐L18) and WT plantlets at a similar status were completely dehydrated for 4 days, then rehydrated for 2 days. Three independent plants of both overexpression line were examined. (b) The RNAi poplars (PalPUB79‐Ri‐L2 and PalPUB79‐Ri‐L12) were more sensitive to drought stress than WT plants. (c–e) Physiological indices, including malondialdehyde (MDA) content (c), electrolyte leakage (EL) (d), chlorophyll content (e), among the overexpression poplars under dehydration conditions. (f–h) MDA content (f), EL (g) and chlorophyll content of RNAi lines under drought stress. Error bars indicate SD (n = 5), **P < 0.01. The experiment was repeated three times with similar results.
In addition, two‐week‐old sterile plantlets were transplanted into solid woody plant medium (WPM) containing 0.2 M mannitol to mimic drought condition, and were maintained under these conditions for a month. The plants overexpressing PalPUB79 showed considerable drought tolerance, with only a limited number of mature leaves turning yellow, while the WT plants experienced severe growth inhibition, with some of the leaves even falling following necrosis (Figure S8a). Moreover, the transgenic PalPUB79‐OE‐L14 and PalPUB79‐OE‐L18 poplars had longer primary roots and lower MDA content than WT plants (Figure S8b, c). The RNAi lines also showed growth inhibition. The RNAi plants had shorter primary roots and higher MDA content than WT plants (Figure S8d–f). These results support that PalPUB79 positively regulates drought tolerance in poplars.
PalPUB79 enhances drought tolerance in an ABA‐dependent manner
ABA signalling is critical in the plant response to drought conditions. In our experiments, PalPUB79 was significantly induced by ABA treatment, suggesting that the improved drought tolerance resulting from PalPUB79 expression might be associated with the ABA signalling pathway. In order to verify this hypothesis, PalPUB79‐OE‐L14 and PalPUB79‐OE‐L18, RNAi and WT plantlets were transplanted into solid WPM containing 5 μM ABA for 1 month. The PalPUB79 overexpression lines were found to be smaller and shorter than WT plants, with the dry weights of PalPUB79‐OE‐L14 and PalPUB79‐OE‐L18 plants less than those observed for WT plants (Figure S9a, b). In contrast, the RNAi plants were larger than the WT plants in terms of both scale and dry weight (Figure S9c, d). These results indicate that PalPUB79 positively regulates hypersensitivity to ABA in poplar.
Fluridone, an inhibitor of ABA biosynthesis, can significantly reduce ABA abundance in plants (González‐Villagra et al., 2019). PalPUB79 overexpression and WT plantlets were transplanted into WPM medium containing mannitol and fluridone for 1 month. The phenotypic differences observed following mannitol treatment disappeared upon the addition of fluridone (Figure 4a), while all of the plants (PalPUB79‐OE‐L14 and PalPUB79‐OE‐L18 plants along with WT plants) showed similar primary root lengths and MDA contents (Figures 4b, c). These results demonstrated that the drought tolerance resulting from PalPUB79 overexpression in poplar is dependent on ABA signalling.
Figure 4.
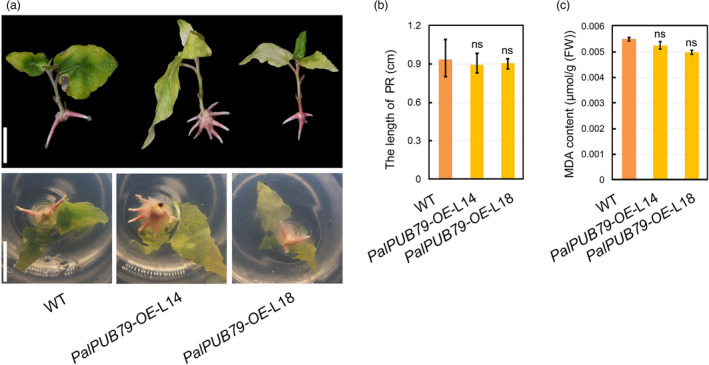
The enhanced drought tolerance of PalPUB79 overexpression poplars was removed by fluridone treatment. (a) PalPUB79 overexpression and WT plantlets were transplanted into the solid WPM containing 100 mM mannitol and 10 μM fluridone for 1 month, with all poplar plantlets were at a similar growth status before treatment. (b) PR length of plantlets. (c) The MDA content in the plantlets. The independent experiment was repeated three times and similar results were obtained.
PalPUB79 influences the expression levels of drought‐associated genes
To determine the influence of PalPUB79 on the poplar transcriptome, RNA‐seq was performed to analyse the expression of drought‐associated genes in PalPUB79 overexpression and RNAi lines. A total of 1366 and 1231 genes in the PalPUB79 overexpression and RNAi lines, respectively, showed significant changes in expression relative to WT plants (Figure 5a and Table S4). Among these genes, 55 were up‐regulated in the PalPUB79 overexpression lines but down‐regulated in RNAi plants (Figure 5a and Table S4). Only one gene (pal_pou29183) showed decreased expression in the PalPUB79 overexpression lines and increased expression in the RNAi lines (Figure 5a and Table S4). Among the 56 genes with differential expression patterns in the overexpression and RNAi plants, 41.07% were involved in drought stress responses (Figure 5b and Table S4). For example, both copies of OSMOTIN 34 (OSM34), which have been shown to be induced by drought stress (Alhaithloul, 2019), demonstrated differential expression patterns in the PalPUB79 overexpression and RNAi poplar plants (Figure 5c, d and Table S4). In addition, LATE EMBRYOGENESIS ABUNDANT 4‐5 (LEA4‐5), which is related to desiccation tolerance (Liu et al., 2010), HISTONE H1‐3 (HIS1‐3), which is induced by dehydration and ABA signalling (Huang et al., 2008), PEROXIDASE 64 (PER64), and RD26, which is critical to the ABA‐mediated dehydration response (Fujita et al., 2004), were significantly up‐regulated in the PalPUB79 overexpression lines but down‐regulated in RNAi poplars (Figure 5c, d and Table S4). These results demonstrate that PalPUB79 positively modulates drought‐associated genes.
Figure 5.
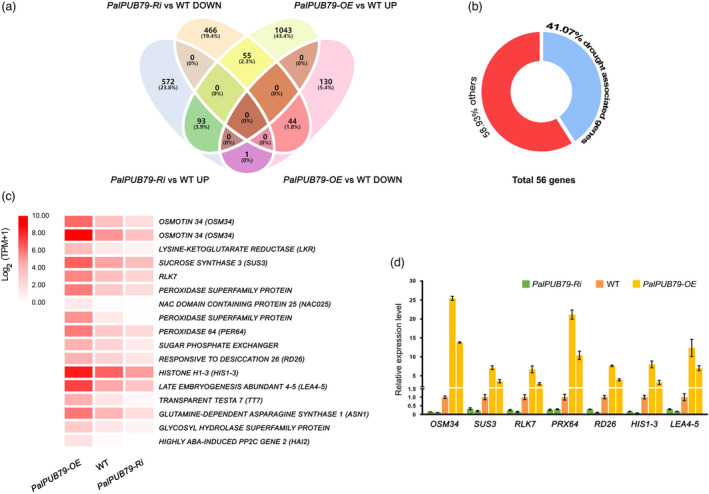
The RNA‐seq and qPCR analysis of PalPUB79 overexpression and RNAi poplars. (a) The Venn diagram indicates which differentially expressed genes overlapped in the PalPUB79 overexpression and RNAi poplars. (b) The doughnut chart showed the proportion of these genes that were related to plant drought response. (c) A heatmap of the expression levels of drought stress‐related genes in WT, PalPUB79 overexpression and RNAi poplars. (d) qPCR verified the expression levels of OSM34, SUS3, RLK7, PRX64, RD26, HIS1‐3 and LEA4‐5 across the different poplar lines. The gene‐specific primers used in the qPCR assay are presented in Table S6. The error bars represent the SD of mean values (n = 3). The internal reference was the ubiquitin (UBQ) gene.
PalPUB79 interacts with PalWRKY77
Each E3 ubiquitin ligase specifically interacts with, and mediates the ubiquitination of, its target protein. In Arabidopsis, exocyst subunit Exo70B1 is the target protein of AtPUB19 (Seo et al., 2016). The two homologues of Exo70B1 in poplar were isolated and termed PalExo70B1.1 and PalExo70B1.2. We performed yeast two‐hybrid assays to examine the interactions between PalExo70B1s and PalPUB79, and found that only PalExo70B1.2 can interact with PalPUB79 (Figure S10a). However, despite mannitol treatment, PalExo70B1.2 showed far lower expression levels than PalExo70B1.1 (Figure S10b). Therefore, PalPUB79 may regulate the ABA pathway in poplar through additional protein targets than just PalExo70B1.2.
A yeast two‐hybrid screening assay with PalPUB79 as the bait was performed to identify other targets of PalPUB79. This assay returned a total of seven proteins; remarkably, the WRKY transcription factor, PalWRKY77, had three hits, which suggested that it is a potential target protein of PalPUB79 (Figure 6a). In a previous study, PalWRKY77 was found to negatively regulate tolerance to salt stress and the ABA response; more specifically, this protein inhibits the expression of PalNAC002 and PalRD26 transcription factors in poplars (Jiang et al., 2020). To determine the interaction between PalPUB79 and PalWRKY77, we re‐constructed the vector expressing an AD‐PalWRKY77 fusion protein, and then subjected this protein to a point‐by‐point yeast two‐hybrid assay. The results indicated that positive transformants harbouring the AD‐PalWRKY77 and BD‐PalPUB79 constructs could grow on screening medium lacking tryptophan (W), leucine (L) and histidine (H); the control could not grow on this screening medium (Figure 6b). In addition, a bimolecular fluorescence complementation (BiFC) assay of the mesophyll protoplast of poplar was used to evaluate the in vivo interaction between PalWRKY77 and PalPUB79. Protoplasts transformed with both nVenus‐PalPUB79 and cCFP‐PalWRKY77 constructs displayed a punctate pattern in the cytoplasm, while no fluorescence signal was detected from the controls (Figure 6c). These results demonstrated that PalPUB79 can interact with PalWRKY77, and that PalWRKY77 might be a target protein of PalPUB79.
Figure 6.
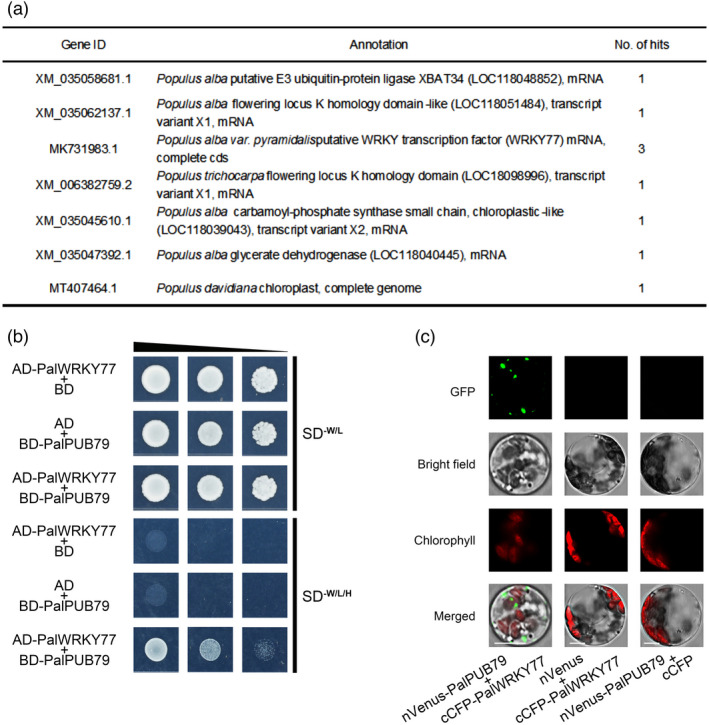
PalPUB79 interacts with PalWRKY77. (a) The list of proteins that interact with PalPUB79 that were screened using a yeast two‐hybrid screening assay. (b) A point‐by‐point yeast two‐hybrid assay provided evidence for the interaction between PalPUB79 and PalWRKY77. The BD and AD empty plasmids with AD‐PalWRKY77 or BD‐PalPUB79 constructs, respectively, served as the negative controls. (c) A bimolecular fluorescence complementation (BiFC) assay was used to demonstrate the in vivo interaction between PalWRKY77 and PalPUB79. The nVenus‐PalPUB79 and cCFP‐PalWRKY77 constructs were co‐transformed into the mesophyll protoplasts of poplar. The empty plasmids with nVenus or cCFP were co‐transformed with cCFP‐PalWRKY77 or nVenus‐PalPUB79, respectively, to serve as negative controls.
PalPUB79 mediates PalWRKY77 degradation by ubiquitination
Before determining whether PalPUB79 can ubiquitinate PalWRKY77 for subsequent degradation, we examined PalWRKY77 abundance in transgenic poplars under mannitol treatment. The results showed that PalWRKY77 levels significantly decreased 2 h after the start of mannitol treatment (Figure 7a); this dynamic could indicate that mannitol treatment induced PalWRKY77 degradation. In addition, the 35S:HA‐PalWRKY77 or 35S:PalPUB79 construct was transiently transformed into Nicotiana benthamiana leaves, which were later harvested for immunoblotting with anti‐HA or anti‐Ub antibodies. High molecular mass bands could be detected by anti‐Ub antibody when the plants were co‐transfected with 35S:PalPUB79 and 35S:HA‐PalWRKY77 (Figure 7b). However, samples harbouring only the 35S:HA‐PalWRKY77 construct did not show significant ubiquitin bands (Figure 7b). Immunoblotting with the anti‐HA antibody revealed a 35 kDa band in samples containing the 35S:HA‐PalWRKY77 construct alone or co‐transfected with 35S:PalPUB79. However, the high molecular mass band was more obvious in samples carrying both the 35S:PalPUB79 and 35S:HA‐PalWRKY77 constructs (Figure 7b). These results indicate that the ubiquitination of PalWRKY77 depends on PalPUB79. In order to investigate whether PalPUB79‐mediated PalWRKY77 degradation is regulated by the UPS, we transiently co‐expressed 35S:HA‐PalWRKY77 and 35S:PalPUB79 constructs in tobacco leaves. We found that PalWRKY77 was degraded, but this degradation could be inhibited by the addition of MG132 (Figure 7c). In addition, bacterially expressed MBP‐PalWRKY77 recombinant protein was incubated with crude extracts prepared from WT and PalWRKY77 overexpression poplars with or without MG132. The recombinant proteins were degraded as the experiment progressed, but the sample with MG132 showed no significant changes in MBP‐PalWRKY77 levels (Figure 7d). In addition, our previous results indicate that PalWRKY77 binds to the W‐box in the promoter of PalRD26 to repress expression in poplars (Jiang et al., 2020). Therefore, we hypothesized that the transcriptional inhibition of PalRD26 by PalWRKY77 should be alleviated when PalPUB79 accumulated. The dual‐luciferase reporter assay indicated that PalRD26 expression decreased relative to the control when the 35S:HA‐PalWRKY77 construct was present, yet PalRD26 expression recovered when both the 35S:HA‐PalWRKY77 and 35S:PalPUB79 constructs were present (Figure 7e). In conclusion, PalWRKY77 is a target protein of PalPUB79, which can mediate the ubiquitination and degradation of PalWRKY77.
Figure 7.
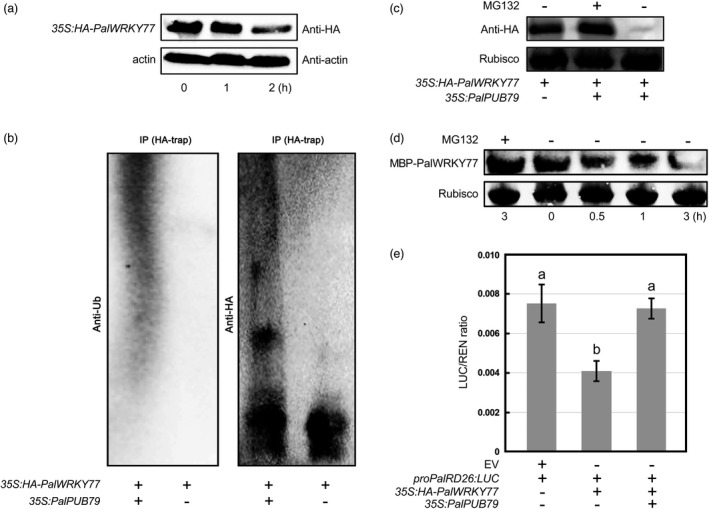
PalPUB79 mediates PalWRKY77 degradation via ubiquitination. (a) 45‐day‐old transgenic poplars expressing the HA‐PalWRKY77 fused protein were irrigated with 300 mM mannitol for 0, 1 and 2 h, after which leaves of the second node were collected for Western blot analysis. The anti‐HA antibody was used to detect the abundance of HA‐PalWRKY77. Actin content, detected using the anti‐actin antibody, served as the internal reference. (b) The 35S: HA‐PalWRKY77 construct, alone or in conjunction with the 35S:PalPUB79 construct, was introduced into Nicotiana benthamiana leaves through agrobacterium‐mediated transformation. Immunoblotting was performed with anti‐HA and anti‐Ub antibodies. (c) PalPUB79‐mediated degradation of PalWRKY77 in tobacco leaves, which involved UPS recruitment, was inhibited by MG132. (d) The cell‐free extract from PalPUB79 overexpression poplar leaves mediated degradation of the bacterially expressed MBP‐PalWRKY77 recombinant protein, and this process was inhibited by MG132. (e) Results of the dual‐luciferase reporter assay, shown as the expression level of firefly luciferase gene (LUC) driven by a 1.5 kb‐length promoter of PalRD26 with or without PalWRKY77 and PalPUB79. Renilla luciferase (REN) activity served as the internal reference. ANOVA was performed in SPSS. Error bars represent the SD of mean values (n = 3), while significant differences (P < 0.05) between groups are indicated by ‘a’ and ‘b’.
PalWRKY77 inhibits PalPUB79 expression
In previous studies, PalWRKY77 overexpression lines have shown decreased expression of PalPUB79 (termed PalPUB19 in Jiang et al., 2020). This suggests that PalPUB79 transcription might be directly down‐regulated by PalWRKY77. A dual‐luciferase reporter assay verified that the co‐expression of 35S:PalWRKY77 and PalPUB79pro:LUC constructs significantly decreased luminescence intensity relative to the controls, and similar results were found upon exposure to ABA or mannitol (Figure 8a). An analysis of the PalPUB79 promoter revealed three W‐boxes in the promoter, which could serve as potential binding sites for PalWRKY77 (Figure 8b). The results of the ChIP‐qPCR indicated that PalWRKY77 binds to the P1 and P2 regions of the PalPUB79 promoter in vivo (Figure 8c), whereas the yeast one‐hybrid assays indicated that PalWRKY77 can bind to one of the W‐boxes, named PW2, but not to PW1 (Figure 8d). Therefore, PalWYKY77 directly down‐regulates PalPUB79 expression by binding to the W‐box (PW2) of the PalPUB79 promoter. In addition, further dual‐luciferase reporter assays showed that the reduced luminescence intensity observed during the co‐expression of 35S:PalWRKY77 and PalPUB79pro:LUC can be reversed by the addition of 35S:PalPUB79 (Figure 8e). These results demonstrate that PalPUB79 can remove the PalWRKY77‐mediated inhibition of PW2 of the PalPUB79 promoter.
Figure 8.
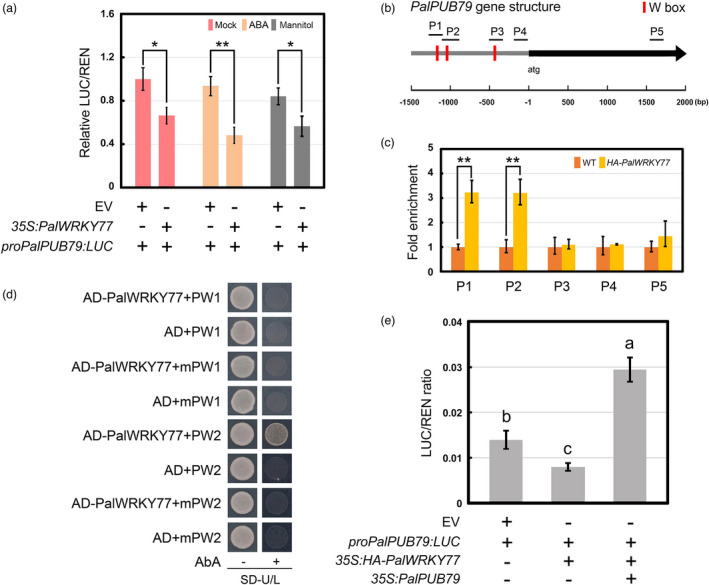
PalWRKY77 negatively regulates PalPUB79 transcription. (a) The results of the dual‐luciferase reporter assay, shown as the expression level of LUC driven by a 1.5 kb‐length promoter of PalPUB79 with or without PalWRKY77. The experiments were performed under normal conditions, mannitol treatment and ABA treatment. Error bars indicate SD (n = 3), *: P < 0.05, **: P < 0.01. (b) The W‐box in the promoter of PalPUB79 and the ChIP‐qPCR results demonstrated that PalWRKY77 binds to the W‐box of the PalPUB79 promoter. WT plants served as the negative controls. P1 to P5 indicate the different regions of the PalPUB79 promoter. Error bars indicated SD (n = 3), **: P < 0.01. All of the primers used in ChIP‐qPCR are listed in Table S6. (c) The yeast one‐hybrid assay results showed how PalWRKY77 binds to the W boxes (PW1 and PW2, the mutant mPW1 and mPW2) in the promoter of PalPUB79. (d) The results of the dual‐luciferase reporter assay, shown as the expression level of LUC driven by a 1.5 kb‐length promoter of PalPUB79 with or without PalWRKY77 and PalPUB79. REN activity served as the internal reference. ANOVA was performed in SPSS. Error bars represent the SD of mean values (n = 3), and significant differences (P < 0.05) between groups are indicated by ‘a’ and ‘b’.
Discussion
Drought stress reduces stem hydraulic conductivity, while severe and long‐term drought conditions decrease the radial growth rate and biomass accumulation (Barber et al., 2000; Choat et al., 2012). Poplars represent a good model system for examining the drought resistance of trees at both physiological and molecular levels (Ragauskas et al., 2006). For example, the desert poplar (Populus euphratica) can endure extremely dry environments because it has a more rapid ABA signalling pathway when compared to drought‐sensitive species (Chen and Polle, 2010). In addition, the molecular mechanisms of ABA signalling during drought stress have been partly elucidated using poplar models (e.g., Jiang et al., 2020). In this study, we identified and characterized a gene involved in the plant drought response, PalPUB79, that encodes a poplar U‐box E3 ubiquitin ligase to up‐regulate drought tolerance in an ABA‐dependent manner. PalPUB79 was found to positively influence the drought response by interacting with PalWRKY77, which has been identified as a repressor of the ABA signalling pathway (Jiang et al., 2020).
Ubiquitination is a process through which a target protein is post‐translationally modified for further degradation by the proteasome (Seok Keun, 2008). E3 Ub ligases interact with target proteins specifically and therefore determine their specificities and functional differences (Marshall and Vierstra, 2015). The P. alba and P. trichocarpa genomes included similar numbers of U‐box genes, which could be divided into three types (ARM REPEAT, KINASES, and UND/ARM REPEAT (Wiborg et al., 2008)) according to the taxonomy of homologous Arabidopsis genes (Tables S1, S2 and Figure S1). To date, many U‐box genes, like AtPUB12, AtPUB13, AtPUB18, AtPUB19, AtPUB46, AtPUB22, AtPUB23 and OsPUB15, have been found to be involved in the plant response to drought stress (Adler et al., 2017; Cho et al., 2008; Kong et al., 2015; Liu et al., 2011; Park et al., 2011; Seo et al., 2016). In poplar, we found that PalPUB79 was significantly induced by salt, mannitol and ABA (Figure 1c–e), with PalPUB79 overexpression enhancing tolerance to drought stress and mannitol treatment in transgenic poplars (Figures 3 and S8). PalPUB79 overexpression also enhanced sensitivity to ABA treatment (Figures 4 and S9). These findings suggest that PalPUB79 positively regulates drought tolerance in an ABA‐dependent manner. We found that the PalPUB79 homologue in Arabidopsis is AtPUB19 (Figure S1), but these two genes have the totally reverse regulation manner (Seo et al., 2016). In fact, PalPUB79 and AtPUB19 only show around 40% sequence identity (Figure S3b), and belong to different phylogenetic clades (Figure 2a). These genes arose from multiple independent duplications (Figure 2a), possibly as a result of a family‐specific WGD or tandem gene duplications during the evolutionary diversification of angiosperm plants. PalPUB79 and all of the orthologues in Salicaceae represent a subclade that is sister to another subclade specific to this family. Therefore, it is likely that PUB79 in Populus and Salix originated from the recent Salicaceae‐specific WGD.
Determining target proteins is crucial to deciphering the function of an E3 ubiquitin ligase. In Arabidopsis, AtPUB22 interacts with, and ubiquitinates, both RPN12a, a subunit of the 26S proteasome, and the exocyst subunit Exo70B2 (Cho et al., 2008; Seo et al., 2016). This evidence indicates that an E3 ubiquitin ligase can have more than one target protein. Moreover, UND, the major structural difference between AtPUB18/AtPUB19 and AtPUB22/AtPUB23, resulted in different target proteins between these two ubiquitin ligases (Seo et al., 2016). Hence, close homologues may have different functions. In poplars, we found that PalPUB79 interacts with PalExo70B1.2, a paralogue of Exo70B1, i.e., a target of AtPUB19 in Arabidopsis (Figure S10a). This interaction may be explained by the functional retention of a specific active site throughout the evolutionary history of these ubiquitin ligases. However, PalExo70B1.2 expression was low during drought stress (Figure S10b). Moreover, the regulation mechanisms governing PalPUB79 and AtPUB19 differ, so PalPUB79 could be expected to target other proteins during drought conditions than AtPUB19. Further experiments identified PalWRKY77 as the target of PalPUB79, i.e., PalWRKY77 was ubiquitinated by PalPUB79 (Figures 6 and 7b). Previous research has shown that PalWRKY77 negatively regulates the poplar response to salt stress and ABA signalling by inhibiting the transcription of PalRD26 (Jiang et al., 2020), which is an ABA‐inducible positive regulator of salt tolerance (Fujita et al., 2010; Hao et al., 2019). For example, PalWRKY77 expression decreased when poplars were exposed to salt stress and ABA (Jiang et al., 2020). In addition, mannitol treatment resulted in the degradation of PalWRKY77 (Figure 7a); this indicates that, in poplars, both PalWRKY77 transcription and PalWRKY77 abundance decrease in response to water deficiency. Interestingly, we found that PalWRKY77 can directly and negatively regulate PalPUB79 transcription (Figure 8), which identified the existence of a negative feedback loop between PalWRKY77 and PalPUB79 that amplifies ABA signalling in poplars. A similar mechanism exists in Arabidopsis; more specifically, ABA‐inducible AtPUB12/AtPUB13 interacts with ABI1, which is a negative co‐receptor of ABA, to mediate ABI degradation (Kong et al., 2015).
We used the results presented in this paper to formulate a mechanism for PalPUB79‐regulated ABA signalling during drought response in poplars (Figure 9). Under normal conditions, endogenous ABA levels are low in poplars and PalWRKY77 represses the transcription of both PalPUB79 and PalRD26 (Figure 9a). However, under dehydration, the rapid accumulation of ABA reduces PalWRKY77 transcription (Jiang et al., 2020) and drives PalPUB79 transcription, consequently mediating PalWRKY77 ubiquitination and degradation. This degradation of PalWRKY77 promotes further PalPUB79 transcription (Figure 9b). In addition, PalWRKY77 degradation also results in increased PalRD26 expression (Figure 9c). The rapid increase in PalRD26 and PalPUB79 expression shifts the poplar from the growth stage to a stress response. Our results thus establish that PalPUB79 participates in a regulation loop for drought tolerance, and the empirical evidence presented in this paper provides valuable insight into the molecular mechanisms underlying ABA signalling in woody plants. In addition, our results also highlight the complex evolution of, and functional diversities among, U‐box genes related to ABA‐dependent stress responses in the annual model plant Arabidopsis and woody poplars.
Figure 9.
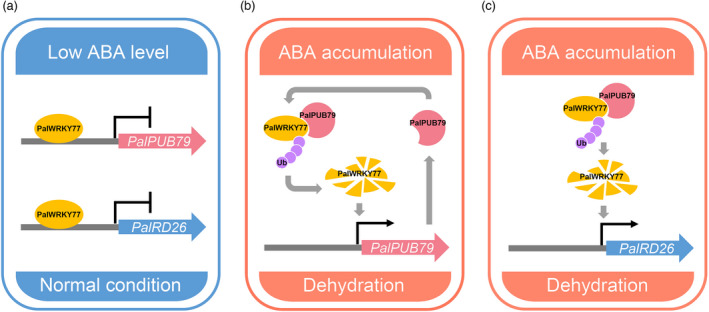
A proposed model for the action mechanism of PalPUB79. (a) PalWRKY77 inhibited PalPUB79 and PalRD26 transcription under normal condition. (b) The feedback regulation of PalPUB79 and PalWRKY77 under drought conditions, i.e., the expression of PalPUB79 removes the PalWRKY77‐mediated inhibition of PalPUB79. (c) PalWRKY77‐mediated inhibition of PalRD26 was relieved by PalPUB79‐mediated ubiquitination of PalWRKY77.
Methods and materials
Plant materials
Populus alba var. pyramidalis plants were cultured in woody plant medium (WPM) supplemented with 0.1 mg/L naphthylacetic acid (NAA) for propagation. Rooted plantlets were transplanted in a greenhouse at 25°C under a 16 h:8 h, light:dark cycle with a light intensity of 4500 lux.
To study PalPUB79 expression across different tissues, two‐month‐old P. alba var. pyramidalis plantlets were collected from the greenhouse and sampled for different tissues, e.g., first expanded leaf (young leaf, YL), the leaf on the fifth node (mature leaf, ML), stem (S), primary root (PR), lateral root (LR), and petiole (P).
Poplar transformation
The coding sequences (CDS) of PalPUB79 were amplified and the PCR product was ligated to the pCXSN expression vector (Chen et al., 2009). The construct was introduced into Populus tomentosa Carr. via agrobacterium‐mediated transformation according to a previous method (Jia et al., 2010).
Treatments
For the salt treatment, two‐month‐old P. alba var. pyramidalis plantlets were watered with 50 ml of 300 mM NaCl solution for 2 h, after which samples were collected for RNA sequencing.
For the mannitol treatment, two‐month‐old P. alba var. pyramidalis plants were watered with 50 ml of 500 mM mannitol solution and harvested 1, 2, 16 and 24 h after treatment for qPCR. In addition, two‐week‐old transgenic P. tomentosa plantlets grown in WPM medium were treated with either 100 mM mannitol only or 100 mM mannitol and 10 μM fluridone.
For the ABA treatment, one‐month‐old poplar plantlets in WPM medium were sprayed with 200 μM ABA solution, including 1ml/L TWEEN 20, for 1, 2, 16 and 24 h. Prior to this, two‐week‐old poplars had been transplanted into WPM medium with 5 μM ABA to test sensitivity to ABA.
For the dehydration treatment, two‐month‐old transgenic and WT poplars were grown in the greenhouse without watering for 5 days, after which they were watered for 2 days.
Phylogenetic analysis and sequence alignment
All of the necessary sequences were downloaded from the TAIR (https://www.arabidopsis.org/index.jsp), NCBI (https://www.ncbi.nlm.nih.gov/), EnsemblPlants (http://plants.ensembl.org/index.html) and JGI (https://phytozome.jgi.doe.gov/pz/portal.html#) websites. The species and gene IDs are listed in Table S6. The phylogenetic trees were constructed in MEGA6 based on the neighbour‐joining method as previously reported (Chen et al., 2020). Sequence alignment was performed in DNAMAN (version 7.0; Lynnon Biosoft, Quebec, Canada).
RNA isolation and qPCR
Total RNA was extracted from fresh poplar tissue using the BIOFIT plant RNA extraction kit (V1.5; Biofit Biotechnologies, Chengdu, China), after which the quantity and quality of the extracted RNA was measured using a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Next, 2 μg of total RNA was reversed transcribed into cDNA with the PrimeScript™ RT reagent kit (TaKaRa, Shiga, Japan) according to the manufacturer’s protocol. This was followed by digestion of the genomic DNA. The qRT‐PCR analysis was performed using Real‐Time Easy™‐SYBR Green Ⅰ (FORE GENE Bio Inc., Chengdu, China). The parameters were 95 °C for 5 min, followed by 39 cycles of 95 °C for 5s and 60 °C for 30 s, which was in accordance with the BIO‐RAD CFX96 Real‐Time System specifications (BIO‐RAD, Hercules, CA). The relative expression of genes was calculated using the (Et)▵CTt/(Er)▵CTr ratio (Wang et al., 2014). All of the gene‐specific primers are shown in Table S6.
RNA‐seq
RNA‐seq data were generated at Novogene Bioinformatics Technology Co. Ltd. (Beijing, China) with an Illumina HiSeq 2000 system (Illumina, San Diego, CA). After processing, we obtained a total of 15.2 GB of clean reads. The Hisat2 algorithm was then used to map the clean reads to the P. alba var. pyramidalis reference genome (Ma et al., 2019). Gene expression abundance was measured using Stringtie v2.1.4, which yielded transcripts per kilobase million (TPM) values (Pertea et al., 2015). Differentially expressed genes (DEGs) were identified by edgeR (Robinson et al., 2010), while Venny2.1 (https://bioinfogp.cnb.csic.es/tools/venny/index.html) was used to create a Venn diagram of DEGs. The DEG heatmaps were generated using EHBIO gene technology resources (http://www.ehbio.com/Cloud_Platform/front/#/).
Yeast two‐hybrid assays and screening
The CDS of genes of interest were cloned and ligated to pGBKT7 and pGADT7 vectors. The constructed bait and prey plasmids were then co‐transformed into the yeast strain AH109 by the PEG/LiAc method (Gietz and Schiestl, 2007). The positive transformants were screened using synthetic dropout (SD) medium without tryptophan (W), leucine (L) and histidine (H). The yeast two‐hybrid screening was performed according to Matchmaker GAL4 Two‐hybrid system 3 & Libraries User Manual (TaKaRa). The cDNA library of P. alba var. pyramidalis was customized using the Clontech kit (TaKaRa).
Yeast one‐hybrid assays
The W‐box with the flanking sequences of the PalPUB79 promoter was repeated three times and introduced into the pHIS2 vector, with the necessary work carried out by Shanghai Sangon Biotechnology (Shanghai, China). PalWRKY77 was introduced into the pGADT7 vector. The method of yeast transformation and the screening medium were identical to what were used in the yeast two‐hybrid assay.
Bimolecular fluorescence complementation (BiFC)
The CDS of PalPUB79 and PalWRKY77 were fused to the CDS of the C‐terminal of CFP (cCFP‐PalWRKY77) and N‐terminal of GFP (nVenus‐PalPUB79), respectively, as previously described (Lee et al., 2008). Mesophyll protoplasts of poplar were isolated from the leaves of three‐week‐old plantlets cultured in WPM solid medium as previously reported (Guo et al., 2012). The constructed plasmids were then introduced into the protoplasts using a transfection method presented by Guo et al., (2012). After 24h in dim light, GFP fluorescence signals were visualized by laser scanning confocal microscope (Leica TCS SP5, Leica Camera AG, Wetzlar, Germany).
Subcellular localization of PalPUB79
The CDS of PalPUB79 were ligated to the pBI221 vector to express a PalPUB79‐GFP fusion protein. The construct or empty pBI221 were introduced into poplar protoplasts and visualized with the same confocal microscope as above.
Dual‐luciferase assay
The 1.5‐kb promoters of PalPUB79 and PalRD26 were inserted into the pGreen II 0800‐LUC vector as reporters, while pCXSN‐HA‐PalWRKY77 and empty pCXSN served as effectors. The constructs were then introduced into leaves of N. benthamiana by agrobacterium‐mediated transient transformation and the renilla (internal control, REN) and firefly (LUC) luciferase signals were detected by a Dual‐luciferase Reporter System (Synergy H1; BioTek, Winooski, VT).
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed according to a previously described protocol (Jiang et al., 2020). Young leaves detached from a two‐month‐old transgenic P. alba var. pyramidalis overexpressing the HA‐PalWRKY77 fusion protein were used as the experimental material (Jiang et al., 2020).
Physiological analysis
Malondialdehyde (MDA) concentrations were quantified as previously described (Wang et al., 2011). Electrolyte leakage (EL) was measured according to a previously established method (Dahro et al., 2016). The dry weight of plant materials was measured after drying in an oven at 60 °C for 3 days.
In vivo protein degradation assay
The recombinant plasmid PCXSN‐HA‐PalWRKY77, with PCXSN‐PalPUB79 or a pCXSN empty vector, was introduced into N. benthamiana leaves by agrobacterium‐mediated transient transformation. For the protein degradation experiments, leaves were harvested after 2 days of darkness and one day of light. The protein samples were prepared according to a previous method (Seo et al., 2016), and the protein degradation patterns were analysed by immunoblotting with anti‐HA (Abcam, Waltham, MA) and anti‐Ubi (Sigma‐Aldrich, St. Louis, MO) antibodies.
In vitro self‐ubiquitination and immunoblot analysis
Recombinant GST‐PalPUB79 protein was expressed in Escherichia coli and purified by affinity chromatography using glutathione sepharose (GE Healthcare, Boston, MA) (Figure S11). The purified fusion proteins GST‐PalPUB79 and His‐Ub, with UBCH5C (E2) and E1 (R&D Systems, Minneapolis, MN), were mixed in ubiquitination reaction buffer (50 mM Tris‐HCl, pH 7.5, 0.5 mM DTT, 2.5 mM MgCl2, 4 mM ATP), then incubated at 30 °C for 1 h. The mixture was boiled with 5× SDS loading buffer for 10 min to terminate the reaction for electrophoresis by 12% SDS‐PAGE gel. The anti‐GST antibody (Sangon Biotech, Shanghai, China) and anti‐Ub antibody (Santa Cruz Biotechnology, Santa Cruz, CA) were used for immunoblotting analysis as described by Lee et al., (2006).
In vivo and in vitro protein degradation assays
To explore in vivo PalWRKY77 degradation, the 35S:HA‐PalWRKY77 construct, with or without the 35S:PalPUB79 construct, was introduced into three‐week‐old tobacco leaves through agrobacterium‐mediated transient transformation. After 2 days of darkness, some of the co‐transformed plants were injected with 50 μM MG132 (AG Scientific, San Diego, CA), while others were injected with water. After one day of growth in normal conditions, the leaves were subjected to grinding with liquid nitrogen for total plant protein extraction. In the immunoprecipitation analysis, the target protein was identified with the anti‐HA antibody, with Rubisco serving as the internal reference.
For the in vitro assay, two‐month‐old poplar leaves overexpressing PalPUB79 were subjected to grinding with liquid nitrogen; the resulting frozen powder was suspended in protein extraction buffer and shaken with a horizontal shaker at 4°C for 1 h. The mixture was centrifuged at 16 000 g at 4°C for 20 min, after which the supernatant was placed in a new tube as the cell‐free protein extract. The MBP‐PalWRKY77 recombinant protein (Figure S11) was incubated with the cell‐free protein extract for 0.5, 1 and 3 h with or without 50 μM MG132 (AG Scientific). All of the reactions were stopped by adding 2×SDS sample buffer, after which SDS‐PAGE electrophoresis was performed. In the immunoprecipitation analysis, the target protein was identified with the anti‐GST antibody, with Rubisco serving as the internal reference.
Conflict of interest
The authors have not declared a conflict of interest.
Author contributions
YJ, TM, JL and HL designed the experiments. ST and NC performed the experiments with the help of BL and SL. DW, FA, LR, YC and JZ analysed the RNA sequencing data. YJ and JL analysed the data and wrote the manuscript.
Supporting information
Figure S1. Phylogenetic analysis of U‐box family members from Arabidopsis, Populus alba var. pyramidalis and P. trichocarpa. The full‐length amino acid sequences of U‐box proteins, including 64 from Arabidopsis, 91 from P. alba var. pyramidalis and 90 from P. trichocarpa, were aligned using Clustal X1.81. The phylogenetic tree was created in MEGA6 using neighbour‐joining with 1000 bootstraps. The U‐box members were divided into three types: KINASES; ARMADILLO/BETA‐CATENIN‐LIKE (ARM) REPEAT; and U‐BOX N‐TERMINAL DOMAIN (UND)/ARM REPEAT. Each type is represented by different colours.
Figure S2. The regulatory elements in the promoter of PalPUB79 were analysed by PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ ). The promoter length was 1.5 kb.
Figure S3. Structural characteristics of PalPUB79, along with the comparison of the amino sequences of PalPUB79 and its homologue AtPUB19. (a) The conserved domains of PalPUB79 were analysed using the Pfam database (https://pfam.xfam.org/ ). (b) Amino acid sequence alignment of PalPUB79 and its homologue AtPUB19 in Arabidopsis by the DNAMAN (version 7.0). The amino acid residues highlighted in black are conserved in the two proteins.
Figure S4. The identification of transgenic poplars overexpressing PalPUB79. (a) PCR using a forward primer of PalPUB79 CDS and a reverse primer of T‐nos showed that the construct was successfully inserted into the poplar genome. (b) The relative expression levels of PalPUB79 among various overexpression lines, as determined by qPCR.
Figure S5. The identification of PalPUB79 RNAi poplars. (a) PCR using specific primers for the spacer sequence indicated that the construct was successfully inserted into the poplar genome. (b) The relative expression levels of PalPUB79 in RNAi poplars, as determined by qPCR.
Figure S6. Stem diameters at the third internode of PalPUB79 overexpression lines and WT plants.
Figure S7. Stem diameters at the third internode of PalPUB79 RNAi lines and WT plants.
Figure S8. The tolerance of PalPUB79 overexpression and RNAi poplars to mannitol treatment. (a) PalPUB79 overexpression and WT poplar plantlets at a similar status were transplanted into WPM medium with mannitol. Three independent plants representing each line were used in the experiment. (b) The PR length of each plantlet was measured. (c) MDA contents across different lines under mannitol treatment. (d) The RNAi and WT poplars in WPM medium with mannitol. (e) The PR lengths of different lines. (f) MDA contents across different lines under mannitol treatment. Error bars indicate SD (n = 5), *: P < 0.05. The experiment was repeated three times.
Figure S9. ABA sensitivity among PalPUB79 overexpression and RNAi poplars. (a) The phenotypes of PalPUB79 overexpression and WT lines before treatment and after 30 d of ABA treatment. (b) The dry weight of poplars. (c) The phenotypes of PalPUB79 RNAi and WT lines before treatment and after 30 d of ABA treatment. (d) The dry weight of the poplars. Error bars indicate SD (n = 3), *: P < 0.05, **: P < 0.01. The experiment was repeated three times.
Figure S10. PalPUB79 interactions with PalExo70B1.1 and PalExo70B1.2 (a) A yeast two‐hybrid assay demonstrated the interactions between PalWRKY77 and PalExo70B1.1 and PalExo70B1.2. A truncated PalPUB79 was used for the determination of the interaction regions. (b) The relative expression levels of PalExo70B1.1 and PalExo70B1.2 in poplars under mannitol treatment. Error bars indicate SD (n = 3).
Figure S11. The Coomassie blue staining of purified MBP‐PalWRKY77 and GST‐PalPUB79 fusion proteins.
Table S1. The U‐box E3 ubiquitin ligases in P. alba var. pyramidalis.
Table S2. The U‐box E3 ubiquitin ligases in P. trichocarpa.
Table S3. The genes differentially expressed under salt treatment compared to controls in P. alba var. pyramidalis.
Table S4. The transcriptome data of PalPUB79 overexpression, RNAi lines and WT.
Table S5. The gene ID used in phylogenetic analysis of Figure 2a.
Table S6. The primers used in this research.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (32071732 and 31700528), National Key Research and Development Program of China (2016YFD0600101) and Fundamental Research Funds for the Central Universities (2020SCUNL207, SCU2019D013).
Tong, S. , Chen, N. , Wang, D. , Ai, F. , Liu, B. , Ren, L. , Chen, Y. , Zhang, J. , Lou, S. , Liu, H. , Liu, J. , Ma, T. and Jiang, Y. (2021) The U‐box E3 ubiquitin ligase PalPUB79 positively regulates ABA‐dependent drought tolerance via ubiquitination of PalWRKY77 in Populus . Plant Biotechnol. J., 10.1111/pbi.13681
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Adler, G. , Konrad, Z. , Zamir, L. , Mishra, A.K. , Raveh, D. and Bar‐Zvi, D. (2017) The Arabidopsis paralogs, PUB46 and PUB48, encoding U‐box E3 ubiquitin ligases, are essential for plant response to drought stress. BMC Plant Biol. 17, 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaithloul, H.A.S. (2019) Impact of combined heat and drought stress on the potential growth responses of the desert grass Artemisia sieberi alba: Relation to biochemical and molecular adaptation. Plants, 8, 416–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber, V.A. , Juday, G.P. and Finney, B.P. (2000) Reduced growth of Alaskan white spruce in the twentieth century from temperature‐induced drought stress. Nature, 405, 668–673. [DOI] [PubMed] [Google Scholar]
- Boudsocq, M. , Barbier‐Brygoo, H. and Lauriere, C. (2004) Identification of nine sucrose nonfermenting 1‐related protein kinases 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana . J. Biol. Chem. 279, 41758–41766. [DOI] [PubMed] [Google Scholar]
- Boyer, J.S. (1982) Plant productivity and environment. Science, 218, 443–448. [DOI] [PubMed] [Google Scholar]
- Chen, N. , Tong, S. , Tang, H. , Zhang, Z. , Liu, B. , Lou, S. , Liu, J. et al. (2020) The PalERF109 transcription factor positively regulates salt tolerance via PalHKT1;2 in Populus alba var. pyramidalis . Tree Physiol. 40, 717–730. [DOI] [PubMed] [Google Scholar]
- Chen, S. and Polle, A. (2010) Salinity tolerance of Populus. Plant Biol. 12, 317–333. [DOI] [PubMed] [Google Scholar]
- Chen, S. , Songkumarn, P. , Liu, J. and Wang, G.L. (2009) A versatile zero background T‐vector system for gene cloning and functional genomics. Plant Physiol. 150, 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, W.‐H. , Endo, A. , Zhou, L.i. , Penney, J. , Chen, H.‐C. , Arroyo, A. , Leon, P. et al. (2002) A unique short‐chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell, 14, 2723–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, S.K. , Ryu, M.Y. , Song, C. , Kwak, J.M. and Kim, W.T. (2008) Arabidopsis PUB22 and PUB23 are homologous U‐Box E3 ubiquitin ligases that play combinatory roles in response to drought stress. Plant Cell, 20, 1899–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat, B. , Jansen, S. , Brodribb, T.J. , Cochard, H. , Delzon, S. , Bhaskar, R. , Bucci, S.J. et al. (2012) Global convergence in the vulnerability of forests to drought. Nature, 491, 752–755. [DOI] [PubMed] [Google Scholar]
- Ciechanover, A. (1998) The ubiquitin‐proteasome pathway: on protein death and cell life. EMBO J. 17, 7151–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahro, B. , Wang, F. , Peng, T. and Liu, J.H. (2016) PtrA/NINV, an alkaline/neutral invertase gene of Poncirus trifoliata, confers enhanced tolerance to multiple abiotic stresses by modulating ROS levels and maintaining photosynthetic efficiency. BMC Plant Biol. 16, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, T. , Park, Y. and Hwang, I. (2015) Abscisic acid: biosynthesis, inactivation, homoeostasis and signalling. Essays Biochem. 58, 29–48. [DOI] [PubMed] [Google Scholar]
- Fujita, M. , Fujita, Y. , Seki, M. , Shuinozaki, K. , Takagi, M. and Shinozaki, K. (2004) Functional analysis of Arabidopsis RD26 gene, a dehydration‐responsive NAC‐related gene. Plant Cell Physiol. 45, S225. [Google Scholar]
- Fujita, M. , Fujita, Y. , Maruyama, K. , Seki, M. , Hiratsu, K. , Ohme‐Takagi, M. , Tran, L.S.P. et al. (2010) A dehydration‐induced NAC protein, RD26, is involved in a novel ABA‐dependent stress‐signaling pathway. Plant J. 39, 863–876. [DOI] [PubMed] [Google Scholar]
- Furihata, T. , Maruyama, K. , Fujita, Y. , Umezawa, T. , Yoshida, R. , Shinozaki, K. and Yamaguchi‐Shinozaki, K. (2006) Abscisic acid‐dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc. Natl. Acad. Sci. USA 103, 1988–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz, R.D. and Schiestl, R.H. (2007) Quick and easy yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2, 35–37. [DOI] [PubMed] [Google Scholar]
- González‐Villagra, J. , Cohen, J. and Reyes‐Díaz, M. (2019) Abscisic acid is involved in phenolic compounds biosynthesis, mainly anthocyanins, in leaves of Aristotelia chilensis plants (Mol.) subjected to drought stress. Physiol. Plant. 165, 855–866. [DOI] [PubMed] [Google Scholar]
- Guo, J. , Morrell‐Falvey, J.L. , Labbe, J.L. , Muchero, W. , Kalluri, U.C. , Tuskan, G.A. and Chen, J.G. (2012) Highly efficient isolation of Populus mesophyll protoplasts and its application in transient expression assays. PLoS One, 7, e44908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao, J. , Buyun, T. and Zhouli, X. (2019) GSK3‐like kinase BIN2 phosphorylates RD26 to potentiate drought signaling in Arabidopsis. Plant J. 100, 923–937. [DOI] [PubMed] [Google Scholar]
- He, K. , Zhao, X. , Chi, X. , Wang, Y. , Jia, C. , Zhang, H. , Zhou, G. et al. (2019) A novel Miscanthus NAC transcription factor MlNAC10 enhances drought and salinity tolerance in transgenic Arabidopsis. J. Plant Physiol. 233, 84–93. [DOI] [PubMed] [Google Scholar]
- Hu, H. and Xiong, L. (2014) Genetic engineering and breeding of drought‐resistant crops. Annu. Rev. Plant Biol. 65, 715–741. [DOI] [PubMed] [Google Scholar]
- Hua, Z.H. and Vierstra, R.D. (2011) The Cullin‐RING ubiquitin‐protein ligases. Annu. Rev. Plant Biol. 62, 299–334. [DOI] [PubMed] [Google Scholar]
- Huang, D.Q. , Wu, W.R. , Abrams, S.R. and Cutler, A.J. (2008) The relationship of drought‐related gene expression in Arabidopsis thaliana to hormonal and environmental factors. J. Exp. Bot. 59, 2991–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, Z. , Gou, J. , Sun, Y. , Yuan, L. , Tang, Q. , Yang, X. , Pei, Y. et al. (2010) Enhanced resistance to fungal pathogens in transgenic Populus tomentosa Carr. by overexpression of an nsLTP‐like antimicrobial protein gene from motherwort (Leonurus japonicus). Tree Physiol. 30, 1599–1605. [DOI] [PubMed] [Google Scholar]
- Jiang, Y. , Tong, S. , Chen, N. , Liu, B. , Bai, Q. , Chen, Y. , Bi, H. et al. (2020) The PalWRKY77 transcription factor negatively regulates salt tolerance and ABA signaling in Populus. Plant J. 105, 1258–1273. [DOI] [PubMed] [Google Scholar]
- Kelley, D.R. (2018) E3 ubiquitin ligases: key regulators of hormone signaling in plants. Mol. Cell. Proteom. 17, 1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, L.Y. , Cheng, J.K. , Zhu, Y.J. , Ding, Y.L. , Meng, J.J. , Chen, Z.Z. , Xie, Q. et al. (2015) Degradation of the ABA co‐receptor ABI1 by PUB12/13 U‐box E3 ligases. Nat. Commun. 6, 8630–8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.H. , Deng, X.W. and Kim, W.T. (2006) Possible role of light in the maintenance of EIN3/EIL1 stability in Arabidopsis seedlings. Biochem. Biophys. Res. Comm. 350, 484–491. [DOI] [PubMed] [Google Scholar]
- Lee, L.Y. , Fang, M.J. , Kuang, L.Y. and Gelvin, S.B. (2008) Vectors for multi‐color bimolecular fluorescence complementation to investigate protein‐protein interactions in living plant cells. Plant Methods, 4, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , Lin, Y.C.J. , Wang, P. , Zhang, B. and Li, W. (2018) Histone acetylation cooperating with AREB1 transcription factor regulates drought response and tolerance in Populus trichocarpa . Plant Cell, 31, tpc.00437.02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Liu, G. , Li, R. , Zou, Y. and Zheng, Y. (2010) Functions of late embryogenesis abundant proteins in desiccation‐tolerance of organisms: a review. Chin. J. Biotechnol. 26, 569–575. [PubMed] [Google Scholar]
- Liu, Y.C. , Wu, Y.R. , Huang, X.H. , Sun, J. and Xie, Q. (2011) AtPUB19, a U‐Box E3 ubiquitin ligase, negatively regulates abscisic acid and drought responses in Arabidopsis thaliana . Mol. Plant 4, 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Yang, T. , Lin, Z. , Gu, B. , Xing, C. , Zhao, L. , Dong, H. et al. (2019) A WRKY transcription factor PbrWRKY53 from Pyrus betulaefolia is involved in drought tolerance and AsA accumulation. Plant Biotechnol. J. 17, 1770–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J.C. , Wan, D.S. , Duan, B.B. , Bai, X.T. , Bai, Q.X. , Chen, N.N. and Ma, T. (2019) Genome sequence and genetic transformation of a widely distributed and cultivated poplar. Plant Biotechnol. J. 17, 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, R.S. and Vierstra, R.D. (2015) Eat or be eaten: the autophagic plight of inactive 26S proteasomes. Autophagy, 11, 1927–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgil, Y. , Shiu, S.H. , Stone, S.L. , Salt, J.N. and Goring, D.R. (2004) A large complement of the predicted Arabidopsis ARM repeat proteins are members of the U‐box E3 ubiquitin ligase family. Plant Physiol. 134, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara, E. and Marion‐Poll, A. (2005) Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 56, 165–185. [DOI] [PubMed] [Google Scholar]
- North, H.M. , De, A.A. , Boutin, J.P. , Frey, A. , To, A. , Botran, L. , Sotta, B. et al. (2010) The Arabidopsis ABA‐deficient mutant aba4 demonstrates that the major route for stress‐induced ABA accumulation is via neoxanthin isomers. Plant J. 50, 810–824. [DOI] [PubMed] [Google Scholar]
- Park, J.J. , Yi, J. , Yoon, J. , Cho, L.H. , Ping, J. , Jeong, H.J. , Cho, S.K. et al. (2011) OsPUB15, an E3 ubiquitin ligase, functions to reduce cellular oxidative stress during seedling establishment. Plant J. 65, 194–205. [DOI] [PubMed] [Google Scholar]
- Pertea, M. , Pertea, G.M. , Antonescu, C.M. , Chang, T.C. , Mendell, J.T. and Salzberg, S.L. (2015) StringTie enables improved reconstruction of a transcriptome from RNA‐seq reads. Nat. Biotechnol. 33, 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragauskas, A. , Williams, C. , Davison, B. , Britovsek, G. , Cairney, J. , Eckert, C. , Frederick, W. et al. (2006) The path forward for biofuels and biomaterials. Science, 311, 484–489. [DOI] [PubMed] [Google Scholar]
- Robinson, M.D. , McCarthy, D.J. and Smyth, G.K. (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics, 26, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohit, J. , Wani, S.H. , Balwant, S. , Abhishek, B. , Dar, Z.A. , Lone, A.A. , Ashwani, P. et al. (2016) Transcription factors and plants response to drought stress: Current understanding and future directions. Front. Plant Sci. 7, 1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, S.H. , Qin, X. and Zeevaart, J.A. (2003) Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol. 131, 1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, D.H. , Ahn, M.Y. , Park, K.Y. , Kim, E.Y. and Kim, W.T. (2016) The N‐terminal UND motif of the Arabidopsis U‐Box E3 Ligase PUB18 Is critical for the negative regulation of ABA‐mediated stomatal movement and determines its ubiquitination specificity for Exocyst Subunit Exo70B1. Plant Cell, 28, 2952–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, M. , Koiwai, H. , Akaba, S. , Komano, T. and Koshiba, T. (2010) Abscisic aldehyde oxidase in leaves of Arabidopsis thaliana . Plant J. 23, 481–488. [DOI] [PubMed] [Google Scholar]
- Seok Keun, C. (2008) Arabidopsis PUB22 and PUB23 are homologous U‐Box E3 ubiquitin ligases that play combinatory roles in response to drought stress. Plant Cell, 7, 1899–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, Y. , Yan, L. , Liu, Z.Q. , Cao, Z. , Mei, C. , Xin, Q. , Wu, F.Q. et al. (2010) The Mg‐chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA‐responsive genes of inhibition. Plant Cell, 22, 1909–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu, F. , Simonneau, T. and Muller, B. (2018) The physiological basis of drought tolerance in crop plants: a scenario‐dependent probabilistic approach. Annu. Rev. Plant Biol. 69, 733–759. [DOI] [PubMed] [Google Scholar]
- Venuprasad, R. , Lafitte, H.R. and Atlin, G.N. (2007) Response to direct selection for grain yield under drought stress in rice. Crop Sci. 47, 285–293. [Google Scholar]
- Wang, H.I. , Chen, J. , Tian, Q. , Wang, S. and Yin, W. (2014) Identification and validation of reference genes for Populus euphratica gene expression analysis during abiotic stresses by quantitative real‐time PCR. Physiol. Plant. 152, 529–545. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Sun, P.P. , Chen, C.L. , Wang, Y. , Fu, X.Z. and Liu, J.H. (2011) An arginine decarboxylase gene PtADC from Poncirus trifoliata confers abiotic stress tolerance and promotes primary root growth in Arabidopsis. J. Exp. Bot. 62, 2899–2914. [DOI] [PubMed] [Google Scholar]
- Wang, N. , Liu, Y.P. , Cong, Y.H. , Wang, T.T. , Zhong, X.J. , Yang, S.P. , Li, Y. et al. (2016) Genome‐wide identification of soybean U‐Box E3 ubiquitin ligases and roles of GmPUB8 in negative regulation of drought stress response in Arabidopsis. Plant Cell Physiol. 57, 1189–1209. [DOI] [PubMed] [Google Scholar]
- Wiborg, J. , O'Shea, C. and Skriver, K. (2008) Biochemical function of typical and variant Arabidopsis thaliana U‐box E3 ubiquitin‐protein ligases. Biochem. J. 413, 447–457. [DOI] [PubMed] [Google Scholar]
- Xiong, L. , Ishitani, M. , Lee, H. and Zhu, J.K. (2001) The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress and osmotic stress‐responsive gene expression. Plant Cell, 13, 2063–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, L. , Lee, H. , Ishitani, M. and Zhu, J.K. (2002) Regulation of osmotic stress‐responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J. Biol. Chem. 277, 8588–8596. [DOI] [PubMed] [Google Scholar]
- Xu, F.Q. and Xue, H.W. (2019) The ubiquitin‐proteasome system in plant responses to environments. Plant Cell Environ. 42, 2931–2944. [DOI] [PubMed] [Google Scholar]
- Zhu, J.K. (2002) Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 53, 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, J.K. (2016) Abiotic stress signaling and responses in plants. Cell, 167, 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Phylogenetic analysis of U‐box family members from Arabidopsis, Populus alba var. pyramidalis and P. trichocarpa. The full‐length amino acid sequences of U‐box proteins, including 64 from Arabidopsis, 91 from P. alba var. pyramidalis and 90 from P. trichocarpa, were aligned using Clustal X1.81. The phylogenetic tree was created in MEGA6 using neighbour‐joining with 1000 bootstraps. The U‐box members were divided into three types: KINASES; ARMADILLO/BETA‐CATENIN‐LIKE (ARM) REPEAT; and U‐BOX N‐TERMINAL DOMAIN (UND)/ARM REPEAT. Each type is represented by different colours.
Figure S2. The regulatory elements in the promoter of PalPUB79 were analysed by PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ ). The promoter length was 1.5 kb.
Figure S3. Structural characteristics of PalPUB79, along with the comparison of the amino sequences of PalPUB79 and its homologue AtPUB19. (a) The conserved domains of PalPUB79 were analysed using the Pfam database (https://pfam.xfam.org/ ). (b) Amino acid sequence alignment of PalPUB79 and its homologue AtPUB19 in Arabidopsis by the DNAMAN (version 7.0). The amino acid residues highlighted in black are conserved in the two proteins.
Figure S4. The identification of transgenic poplars overexpressing PalPUB79. (a) PCR using a forward primer of PalPUB79 CDS and a reverse primer of T‐nos showed that the construct was successfully inserted into the poplar genome. (b) The relative expression levels of PalPUB79 among various overexpression lines, as determined by qPCR.
Figure S5. The identification of PalPUB79 RNAi poplars. (a) PCR using specific primers for the spacer sequence indicated that the construct was successfully inserted into the poplar genome. (b) The relative expression levels of PalPUB79 in RNAi poplars, as determined by qPCR.
Figure S6. Stem diameters at the third internode of PalPUB79 overexpression lines and WT plants.
Figure S7. Stem diameters at the third internode of PalPUB79 RNAi lines and WT plants.
Figure S8. The tolerance of PalPUB79 overexpression and RNAi poplars to mannitol treatment. (a) PalPUB79 overexpression and WT poplar plantlets at a similar status were transplanted into WPM medium with mannitol. Three independent plants representing each line were used in the experiment. (b) The PR length of each plantlet was measured. (c) MDA contents across different lines under mannitol treatment. (d) The RNAi and WT poplars in WPM medium with mannitol. (e) The PR lengths of different lines. (f) MDA contents across different lines under mannitol treatment. Error bars indicate SD (n = 5), *: P < 0.05. The experiment was repeated three times.
Figure S9. ABA sensitivity among PalPUB79 overexpression and RNAi poplars. (a) The phenotypes of PalPUB79 overexpression and WT lines before treatment and after 30 d of ABA treatment. (b) The dry weight of poplars. (c) The phenotypes of PalPUB79 RNAi and WT lines before treatment and after 30 d of ABA treatment. (d) The dry weight of the poplars. Error bars indicate SD (n = 3), *: P < 0.05, **: P < 0.01. The experiment was repeated three times.
Figure S10. PalPUB79 interactions with PalExo70B1.1 and PalExo70B1.2 (a) A yeast two‐hybrid assay demonstrated the interactions between PalWRKY77 and PalExo70B1.1 and PalExo70B1.2. A truncated PalPUB79 was used for the determination of the interaction regions. (b) The relative expression levels of PalExo70B1.1 and PalExo70B1.2 in poplars under mannitol treatment. Error bars indicate SD (n = 3).
Figure S11. The Coomassie blue staining of purified MBP‐PalWRKY77 and GST‐PalPUB79 fusion proteins.
Table S1. The U‐box E3 ubiquitin ligases in P. alba var. pyramidalis.
Table S2. The U‐box E3 ubiquitin ligases in P. trichocarpa.
Table S3. The genes differentially expressed under salt treatment compared to controls in P. alba var. pyramidalis.
Table S4. The transcriptome data of PalPUB79 overexpression, RNAi lines and WT.
Table S5. The gene ID used in phylogenetic analysis of Figure 2a.
Table S6. The primers used in this research.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


