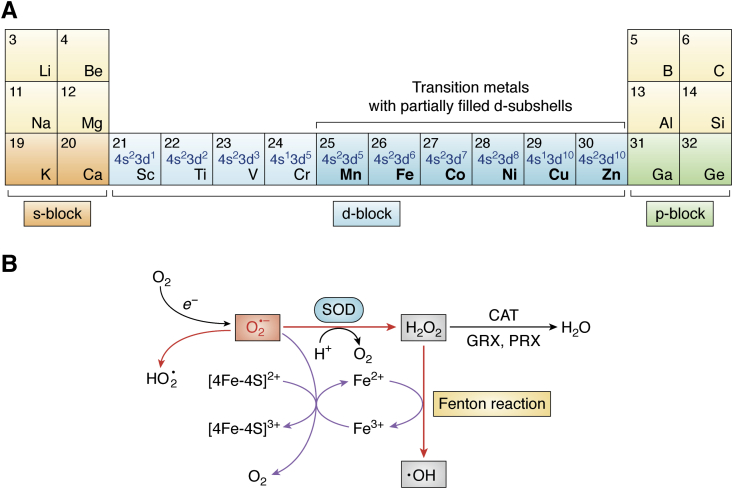
Figure 1.
Transition metals and oxidative stress.A, transition metals. A section of the periodic table showing s-block (orange), d-block (cyan), and p-block (green) chemical elements. Transition metals of the first row, characterized by partially filled d-subshells, are shown in dark cyan squares in bolded black lettering. Zn (which possesses a complete d-subshell) is also shown in this group. The electronic structures of the d-block elements are shown in blue lettering. B, reactions of the superoxide anion; iron and redox cycling. Negatively charged free radical superoxide (O2•−, shown in red lettering) is the product of one-electron (e−) reduction of dioxygen (O2). Upon protonation, O2•− can form the hydroperoxyl radical (HO2•). Superoxide dismutase (SOD, cyan oval) catalyzes the dismutation (disproportionation) of O2•−, thereby generating O2 and hydrogen peroxide (H2O2). H2O2 is converted to H2O by various antioxidant enzymes, such as catalases (CAT), glutathione peroxidases (GPX), and peroxiredoxins (PRX). Redox-active Fe2+ ions are oxidized by H2O2, generating highly reactive hydroxyl radicals (OH•) and Fe3+ through the Fenton reaction. Fe3+ can be reduced to Fe2+ by O2•−, resulting in redox cycling (purple arrows). By itself, O2•− can reduce Fe3+ to Fe2+ within iron–sulfur cluster proteins, resulting in enzyme inactivation and accumulation of Fe2+, which further powers Fenton chemistry. Modified from Ref. (271).