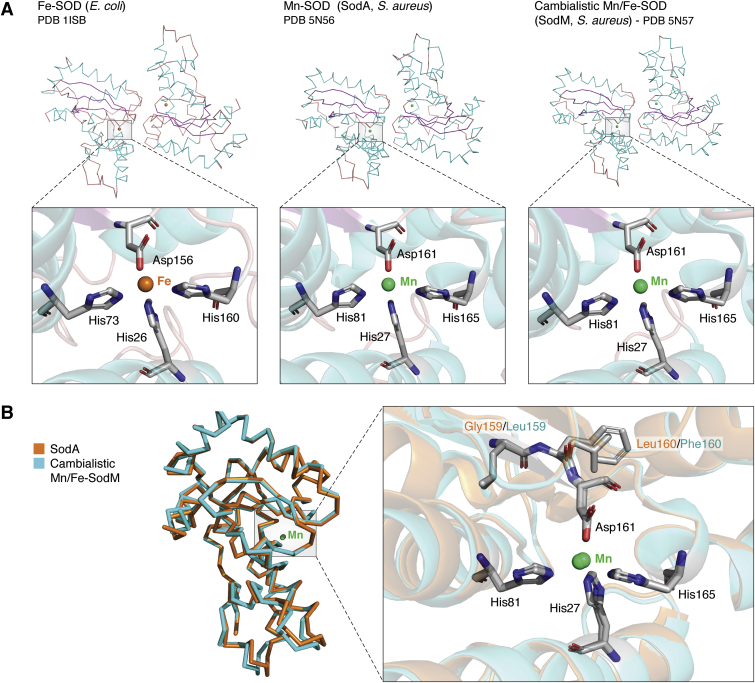
Figure 4.
Structural properties of Mn-specific, Fe-specific, and cambialistic Mn/Fe-SODs.A, comparison of the crystal structures (top) and the metal-bound active sites (bottom) of Fe-SOD from Escherichia coli, Mn-SOD (SodA) and cambialistic Mn/Fe-SOD (cam-SOD, SodM) from Staphylococcus aureus. Metal ions (shown as colored spheres; orange for iron ion and green for manganese ion) are coordinated by three histidine (His) residues and one aspartic acid (Asp) residue and a solvent molecule (not shown). The figure is generated using PyMol; Protein Data Bank IDs are indicated in the figure. B, superimposed ribbon representation of Mn-specific SodA (orange) and cambialistic Mn/Fe-SodM (cyan) monomer structures from Staphylococcus aureus is shown on the left. Superimposed structures of active centers of SodA and SodM are shown on the right. Four residues from the primary coordination sphere (His27, His81, Asp161, and His165, black lettering) coordinate a metal ion (Mn is shown as a green sphere). Two residues from the secondary coordination sphere (SodA: Gly159, Leu160, orange lettering; SodM: Leu159, Phe160, cyan lettering) provide cambialistic properties to SodM. SOD, superoxide dismutase.