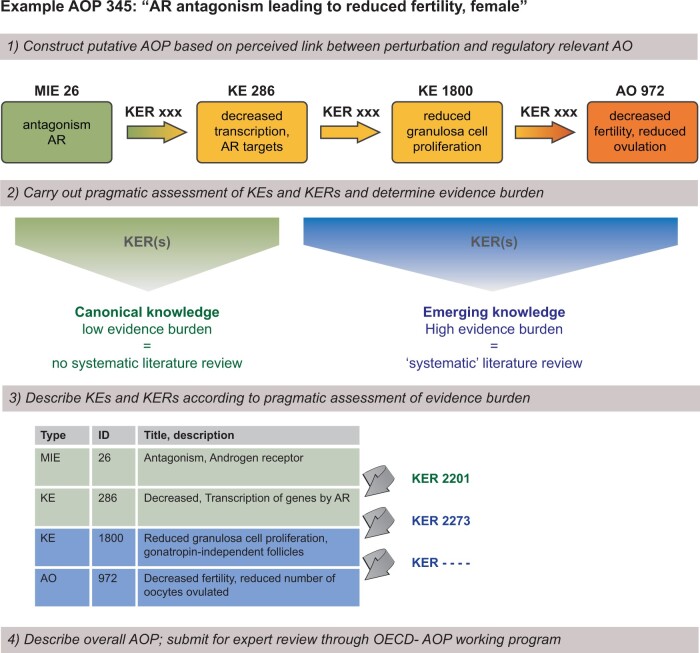
Figure 2.
Developing AOPs by adopting pragmatic approaches to elaborating KERs. AOPs comprise a series of KEs from an MIE through to an AO that are linked by KERs. Although the KEs are important building blocks, KERs represent the most elaborate and important information for any AOP to be of regulatory use as it provides the causal link between chemical perturbation and adverse effects in intact organisms. Taken together, all KEs and KERs that form an AOP make up a substantial body of supporting knowledge, with a single KER easily comprising a large database of articles that itself could fill an extensive review article in a scientific journal. To lessen the burden on both developers and reviewers of AOPs under development, KERs should be substantiated differently depending on the level of general acceptance of the causal relationship in question. In instances where the knowledge is considered “text-book,” or canonical, there should be no need for an extensive review process, but rather a reliance on pre-existing literature reviews. When the knowledge is not considered canonical, systematic literature approach should be adopted before being subjected to peer review and ultimately endorsement. Abbreviations: AO, adverse outcome; AOP, adverse outcome pathway; KE, key event; KER, key event relationship; MIE, molecular initiating event.