Fig. 1.
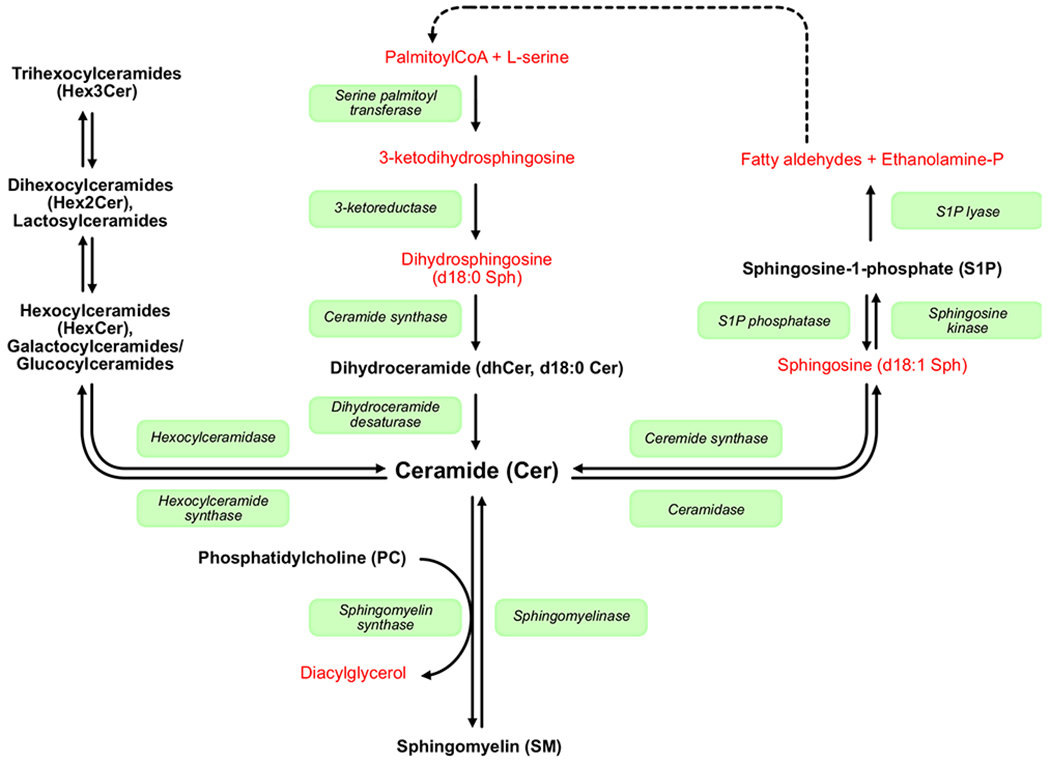
Simplified scheme of sphingolipid biosynthesis. Selected enzymes participating in the sphingolipid metabolism are shown in boxes, and lipids analyzed in this study are indicated in black bold letters. De novo sphingolipid synthesis is initiated by the condensation of fatty acyl-CoA and l-serine. Palmitoyl-CoA (16:0) is the favored substrate for this reaction, but other fatty acyl-CoAs, primarily myristoyl-CoA (14:0) and stearoyl-CoA (18:0), may also be used, as well as other amino acids. From the point of ceramide, the synthesis pathways branch out creating a wealth of complex sphingolipids, differing in their lipid backbones and head groups. The only exit for sphingolipids from this tree is by the activity of S1P lyase on sphingosine-1-phosphate, producing ethanolamine phosphate and fatty aldehydes. The fatty aldehydes can be recycled through a series of enzymatic steps (not shown) to reenter the de novo pathway or be used in other metabolic processes.
