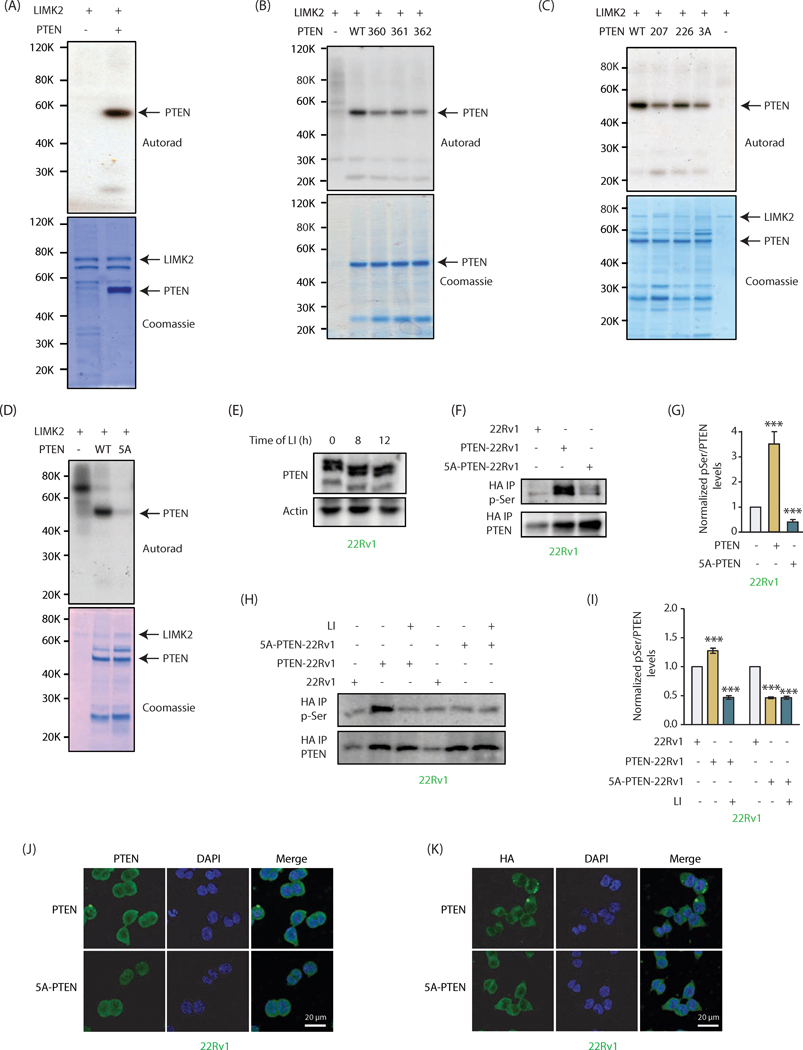
Figure 4:
PTEN is directly phosphorylated by LIMK2 at five sites (A) LIMK2 directly phosphorylates PTEN. Lane 1 contains [32P]ATP and LIMK2 and lane 2 contains 6x-His-PTEN, LIMK2 and [32P]ATP. Kinase reaction was conducted for 30 minutes. The lower panel shows LIMK2 and PTEN Coomassie staining of the same gel. (B) LIMK2 phosphorylates PTEN at S360, S361 and S362 sites. WT and single mutants of PTEN at S360, S361 and S362 sites were subjected to in vitro kinase assay using LIMK2 and [32P]ATP. (C) LIMK2 phosphorylates PTEN at S207, S226 and 3A sites (S360/S361/S362). The corresponding phospho-resistant single mutants (S207A, S226A) and triple mutant S3A (360A, S361A and S362A) were generated and subjected to in vitro kinase assay using LIMK2. The top panel shows autoradiography, and lower panel shows LIMK2 and PTEN Coomassie stains. (D) LIMK2 phosphorylates PTEN at only above-mentioned five sites (S207, S226, S360, S361 and S362), as the corresponding 5A-phospho-resistant mutant did not show any phosphorylation. (E) Mobility shift analysis of PTEN phosphorylation in intact cells. LIMK2 was inhibited in 22Rv1 cells using LIMK2 inhibitor (LI, 10 μM) for 8h and 12h and lysates were run on 10% SDS-PAGE gel followed by immunoblotting using PTEN antibody. (F) Ectopically expressed WT and 5A-PTEN were immunoprecipitated using HA antibody and assayed for phospho-serine levels. (G) Quantification of blots obtained from three independent experiments in part F reveal a significant loss of phosphorylation in case of the 5A mutant, ***P<0.0005. (H) Phospho-serine levels were also assayed with and without inhibition of LIMK2 (10 μM LI, 12h) in both PTEN-22Rv1 and 5A-PTEN-22Rv1 cells. (I) Quantification of data obtained for part H shows minimal phosphorylation of 5A-PTEN. Furthermore, phospho-levels of 5A-PTEN remain unchanged by LIMK2 inhibition confirming that these are the only sites of phosphorylation by LIMK2. Graph represents data obtained from three independent experiments. These are shown as mean ± SEM with ***P <0.0005 compared to control cells. (J) Subcellular localization of PTEN in PTEN-22Rv1 and 5A-PTEN-22Rv1 cells. (K) PTEN and 5A-mutant showed similar subcellular localization when analyzed using HA antibody.