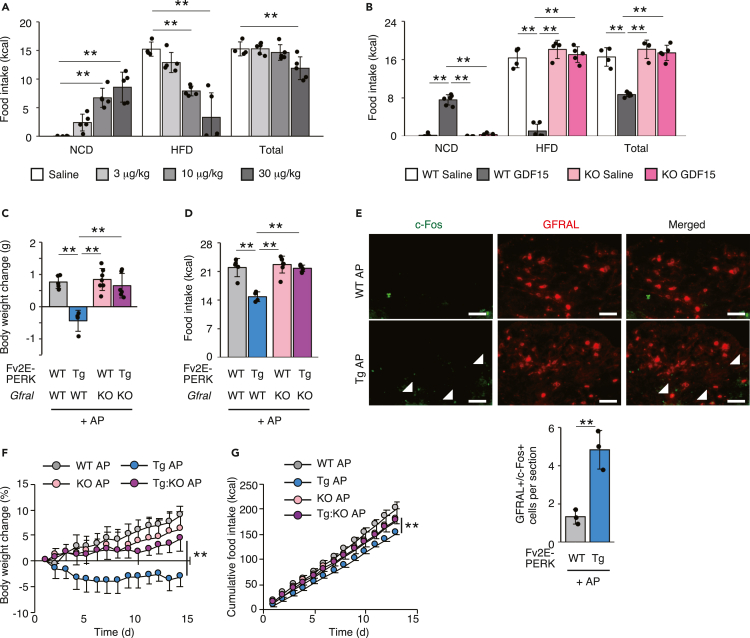
Figure 5.
GFRAL is responsible for inhibiting the intake of the HFD through a mechanism mediated by phosphorylated eIF2α and GDF15
(A) Food intake measured for 12 h during the two-diet choice experiment (NCD and HFD) in WT mice treated with the indicated doses of GDF15 (0: n = 4, 0.003: n = 5, 0.01: n = 5, 0.03: n = 5).
(B) Food intake measured for 12 h during the two-diet (NCD and HFD) choice experiment in WT or Gfral-deficient (KO) mice treated with 0.03 mg/kg GDF15 (WT saline: n = 4, WT GDF15: n = 5, KO saline: n = 4, KO GDF15: n = 5).
(C) Changes in BW 24 h after the AP injection in WT, Tg, Gfral KO, and Tg:Gfral KO mice fed the HFD (WT AP: n = 5, Tg AP: n = 4, KO AP: n = 7, Tg:KO AP: n = 6).
(D) Food intake measured for 24 h in AP-injected WT, Tg, Gfral KO, and Tg:Gfral KO mice fed the HFD (WT AP: n = 5, Tg AP: n = 4, KO AP: n = 7, Tg:KO AP: n = 6).
(E) Representative photograph of area postrema immunostained for the c-Fos and GFRAL from WT and Tg mice treated with AP after 24 h (scale bar, 50 μm) (WT AP: n = 3, Tg AP: n = 3). Arrow heads show c-Fos and GFRAL double-positive cells. The graph below shows quantification of c-Fos and GFRAL double-positive cells in section.
(F) Changes in BW of the AP-injected WT, Tg, Gfral KO, and Tg:Gfral KO mice fed the HFD (WT AP: n = 4, WT AP: n = 5, KO AP: n = 5, Tg:KO AP: n = 6).
(G) Cumulative food intake of AP-injected WT, Tg, Gfral KO, and Tg:Gfral KO mice fed the HFD (WT AP: n = 4, WT AP: n = 5, KO AP: n = 5, Tg:KO AP: n = 6).
All data are presented as means ± SD. Unpaired two-tailed Student's t tests were used to analyze the data presented in (E). One-way ANOVA followed by Holm-Sidak multiple comparisons tests were used to analyze the data presented in (A–D). Two-way ANOVAs were used to analyze the data presented in (F and G) (Tg AP and Tg:KO AP). ∗∗P < 0.01.