Figure EV6. MLKL ubiquitylation antagonizes necroptosis.
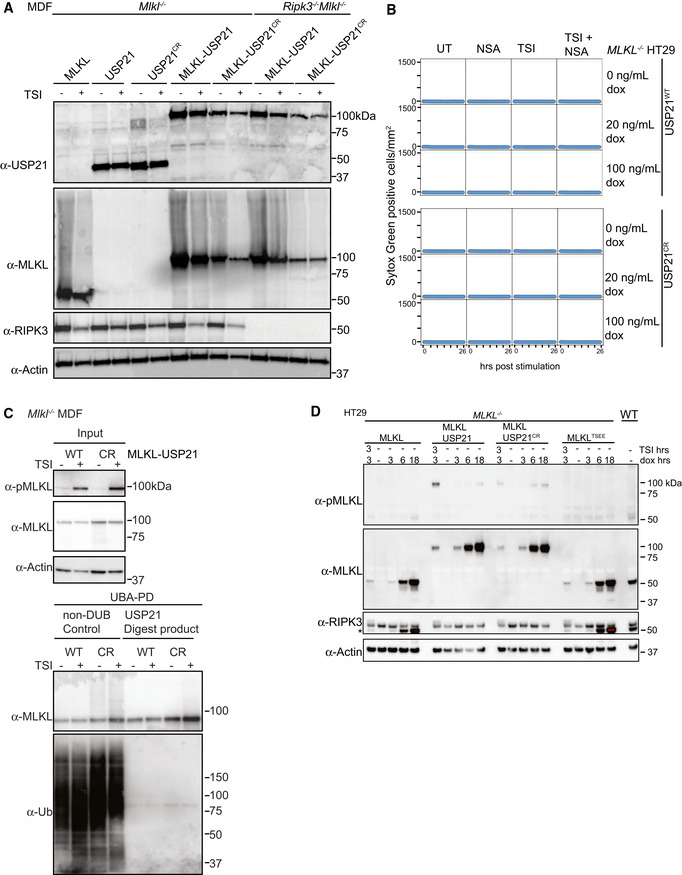
- Mouse MLKL, USP21, USP21C221R, MLKL‐USP21 and MLKL‐USP21C221R fusions were inducibly expressed in Mlkl −/− MDFs and Ripk3 −/− Mlkl −/− MDFs as indicated, following doxycycline addition (20 ng/ml) for 6 h ± TSI. Representative of two independent experiments.
- MLKL −/− HT29 cells stably transfected with doxycycline‐inducible constructs encoding human USP21 and human USP21C221R were treated with doxycycline, NSA (1 μM), TSI or combinations thereof (added simultaneously). Sytox Green‐positive cells were quantified in real time by live cell imaging. Representative of two independent experiments.
- Mouse MLKL‐USP21 and MLKL‐USP21C221R fusions were inducibly expressed in Mlkl −/− MDFs by doxycycline (10 ng/ml) for 8 h with addition of a necroptotic stimulus (TSI) for 3 h, followed by UBA pulldown and USP21 digestion. Antibody (D6E3G, Cell Signaling Technology) was used here to detect MLKL phosphorylation. Representative of two independent experiments.
- MLKL −/− HT29 cells were stably transfected with indicated doxycycline‐inducible MLKL alleles (phospho‐mimetic human MLKL mutant T357E/S358E indicated as MLKLTSEE) and treated with doxycycline (100 ng/ml) ± TSI (added simultaneously). A residue band from MLKL blot is indicated by an asterisk in RIPK3 blot due to reprobing. Representative of three independent experiments.
Data information: TSI is used as a necroptotic stimulus.
Source data are available online for this figure.
