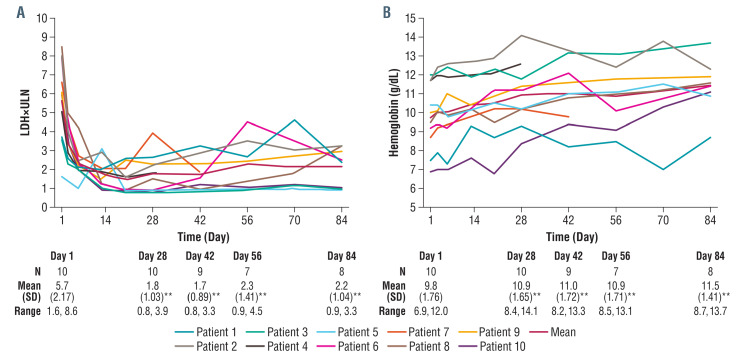
Figure 2.
Effect of danicopan on lactate dehydrogenase and hemoglobin levels. (A) Change in lactate dehydrogenase (LDH) concentration from baseline (day 1) to day 28 was the primary efficacy endpoint. LDH reduction per patient is shown here including the reduction from a mean value of 5.7±2.17 times upper limit of normal (ULN) at baseline to 1.8±1.03 times ULN at day 28 (P<0.001) demonstrating achievement of the primary endpoint. A significant mean LDH reduction from baseline was sustained throughout the study up to day 84 (2.2±1.04 times ULN; P<0.001). (B) Per patient effects on hemoglobin with a mean group increase from 9.8 g/dL at baseline (day 1) (range, 6.9 to 12.0 g/dL) to 10.9 g/dL at day 28 (range, 8.4 to 14.1 g/dL; P<0.005), and 11.5 g/dL at day 84 (range, 8.7 to 13.7 g/dL, P<0.005). Note, patient 3 received a protocol waiver to enter the study despite the <12 g/dL hemoglobin inclusion criterion. For the sponsor and site investigator, the hemoglobin level did not represent a clinically meaningful difference relative to the threshold in the protocol and the patient had significant hemolysis, as evidenced by LDH values. **P<0.005; SD:standard deviation.