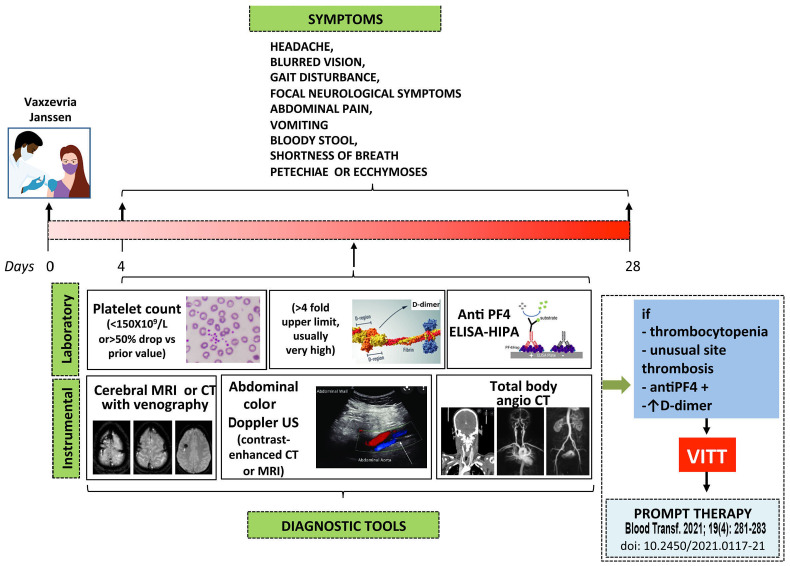
Figure 3.
A suggested clinical surveillance and diagnostic approach to suspected vaccine-induced immune thrombotic thrombocytopenia. Subjects receiving the Vaxzevria and the Janssen vaccines who develop new onset headache, especially if severe or with unprecedented characteristics, and/or associated with other clinical manifestations (blurred vision, gait disturbance, focal neurological symptoms and/or abdominal pain, vomiting, bloody stool, shortness of breath, petechiae or ecchymoses) should be referred for immediate laboratory evaluation (platelet count and D-dimer measurement). If thrombocytopenia is detected they should undergo anti-PF4 antibody testing, imaging and evaluation for cerebral vein sinus thrombosis, splanchnic vein thrombosis or pulmonary embolism. If confirmed, therapy for vaccine-induced immune thrombocytopenic thrombosis (VITT) should be immediately started according to the statement from the Italian Society for the Study of Haemostasis and Thrombosis (SISET)13 Although almost one quarter of the reported patients with VITT in whom unfractionated or low-molecular weight heparin was used apparently responded well to treatment (Table 2), subjects positive for anti-PF4 antibodies, as determined by a heparin-induced platelet aggregation (HIPA) test and/or enzyme-linked immunosorbent assay, or who have not been tested should, for prudence, be treated with alternative anticoagulants, until new information becomes available. In subjects in whom these anti-PF4 antibodies do not cross-react with heparin, as shown by a HIPA test in the presence of a low concentration of heparin, the use of heparin as anticoagulant may be allowed. ELISA: enzyme-linked immunosorbent assay; MRI: magnetic resonance imaging; CT: computed tomography; US: ultrasound.