FIGURE 2.
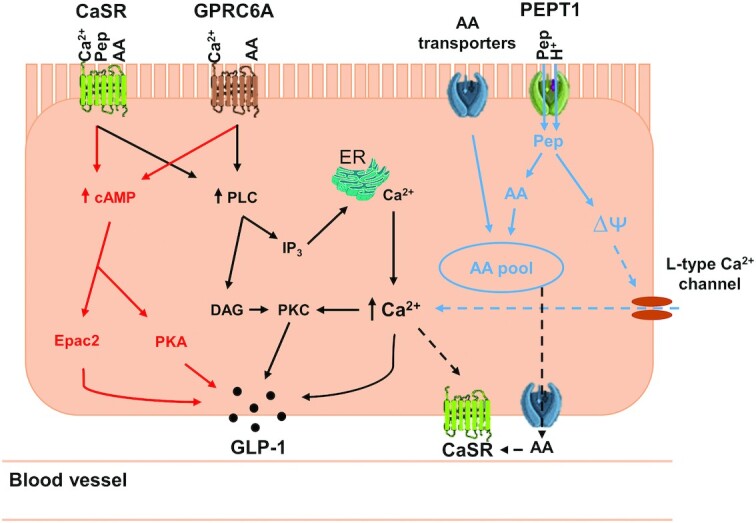
The potential putative mechanisms of calcium and protein synergy inducing GLP-1 secretion. Amino acids and peptides are sensed by CaSR (amino acids and peptides) and GPRC6A (amino acids only). The ability of CaSR to bind both peptides and amino acids is dependent on the presence of calcium, whereas GPRC6A also contains a calcium-binding site. This binding leads to 2 downstream signaling pathways involving cAMP (red) and phosphatidylinositol (black), leading to GLP-1 exocytosis. PEPT1 mediates the transport of peptides into the cell and subsequently causes membrane depolarization (potentially leading to calcium influx via L-type Ca2+ channels—blue dashed arrows) and ultimately GLP-1 exocytosis. Amino acids that enter the cell via amino acid transporters, including B˚AT1, and peptides that are broken down, join the amino acid intracellular pool. Intracellular amino acids and peptides transported out of the cell might also be sensed by CaSR localized on the basolateral membrane (black dashed arrows), which similarly triggers the signaling pathways highlighted in red and black. AA, amino acids; B˚AT1, sodium-dependent neutral amino acid transporter; CaSR, extracellular calcium-sensing receptor; DAG, diacylglycerol; Epac2, exchange protein directly activated by cAMP 2; ER, endoplasmic reticulum; GLP-1, glucagon-like peptide 1; GPRC6A, G-protein–coupled receptor class C 6A; IP3, inositol 1,4,5-triphosphate; Pep, peptides; PEPT1, peptide transporter 1; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C; ∆ᴪ, membrane depolarization.
