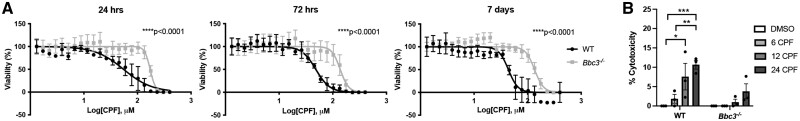
Figure 3.
Bbc3 −/− primary cortical neurons are resistant to chlorpyrifos (CPF)-induced cell death. (A) IC50 curves for WT and Bbc3−/− primary cortical neurons treated 24 or 72 h, or 7 days with [1–400 μM] CPF. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays were performed to assess viability. All data were normalized to vehicle-treated (DMSO) controls. Mean percent viability is shown for 3 biologic replicates, error bars represent standard deviation after log-transformation of CPF concentrations. p Values were determined using nonlinear regression. We saw a significantly higher IC50 value of CPF-treated Bbc3−/− neurons (172.3 μM) compared with WT neurons (54.98 μM) (****p < .0001) at 24 h, at 72 h (Bbc3−/−, 137.5 μM; WT, 48.31 μM; ****p < .0001), and at 7 days (Bbc3−/−, 134.9 μM; WT, 43.75 μM; ****p < .0001). B, A lactate dehydrogenase assay was performed on media of WT and Bbc3−/− primary cortical neurons exposed to 50 µM CPF for 6, 12, or 24 h or equivalent vehicle (DMSO) for 24 h. With increasing time exposed to the approximate WT IC50 of CPF (approximately 50 µM), there was an increase in cytotoxicity in WT cultures (2-way ANOVA; **p = .0027 (genotype), ***p = .0008 (treatment), nsp = .0936 (genotype × treatment); WT 24 DMSO to WT 12 CPF, *p = .0140; WT 24 DMSO to WT 24 CPF, ***p = .0008). An increase in cytotoxicity was seen from 6 to 24 h in WT cultures as well (WT 6 CPF to WT 12 CPF, **p = .0043). No significant difference in cytotoxicity was observed in identically exposed-Bbc3−/− primary cortical neurons. Error bars represent SEM.