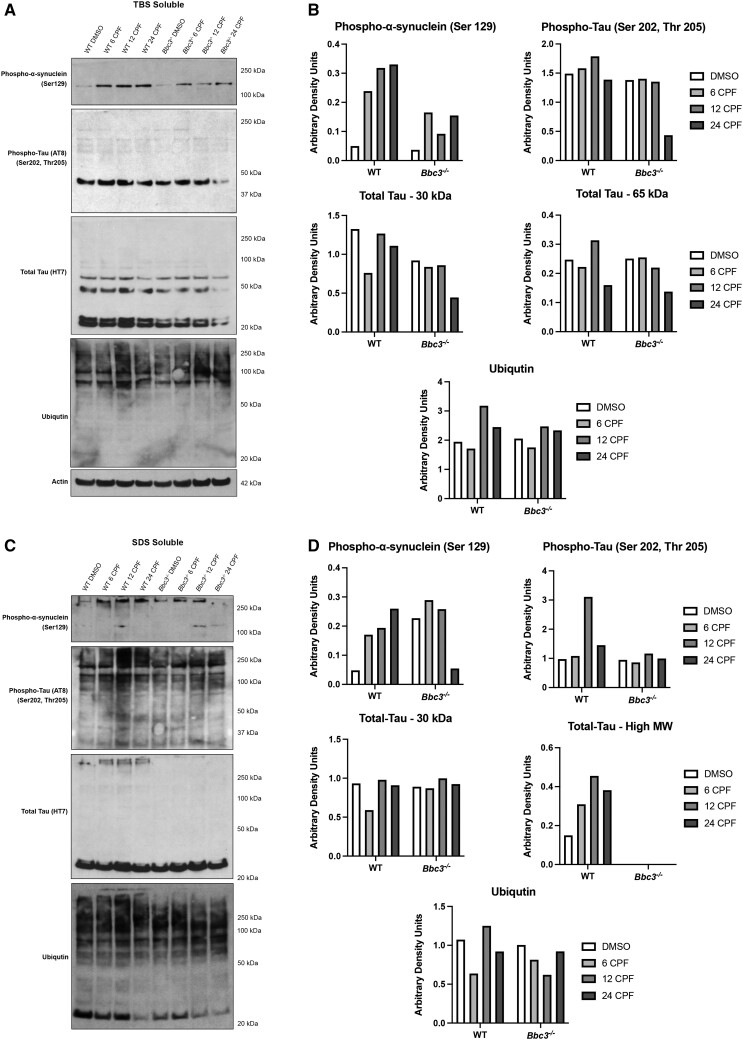
Figure 6.
Chlorpyrifos (CPF) exposure results in aberrant protein accumulation. WT and Bbc3−/− embryonic cortical neurons (E14.5–E16.5) were exposed to vehicle (DMSO) or 50 µM CPF for 6, 12, or 24 h. Lysate was harvested and protein solubility assay was performed by conducting ultracentrifugation using buffers of increasing stringency. Lysates from the tris-buffered saline (TBS) fraction (least stringent) and SDS fraction (most stringent) were analyzed using SDS-PAGE followed by immunoblotting. Membranes were probed with antibodies for proteins known to misfold and aggregate in the neurodegenerative disease process including anti-phospho-alpha-synuclein, anti-phospho-tau, anti-tau, and anti-ubiquitin. A and B, Within the TBS soluble fraction, CPF exposure increases high molecular weight (MW) phospho-alpha-synuclein (Ser129) in CPF-exposed WT cultures and, to a lesser extent, in CPF-treated Bbc3−/− cultures. C and D, In the SDS soluble fraction, high MW phospho-alpha-synuclein (Ser129), high MW phospho-tau (Ser202, Thr205), and high MW total tau is increased with CPF treatment in WT cultures, that is either slightly attenuated, or completely absent, in Bbc3−/− cultures. There does not appear to be any major differences in ubiquitination across fractions, genotypes, or treatments. (B and D) Densitometry was performed and data normalized to actin in the TBS fraction. SDS fractions vary based on the presence of insoluble protein in this original sample. Data represent 1 biologic replicate, but as noted in the Methods, each biologic replicate consists of pooled neurons from at least 6 embryos.